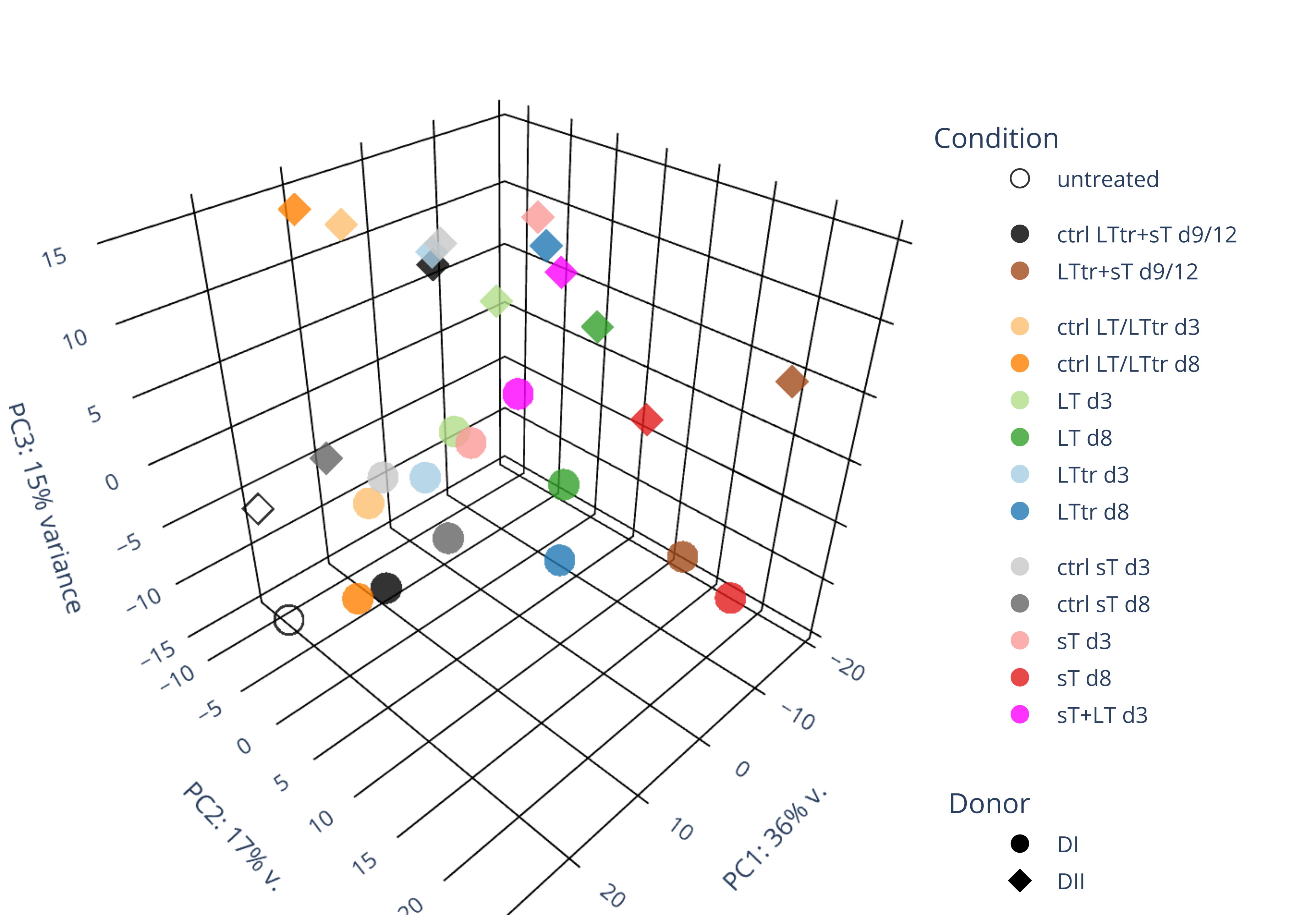
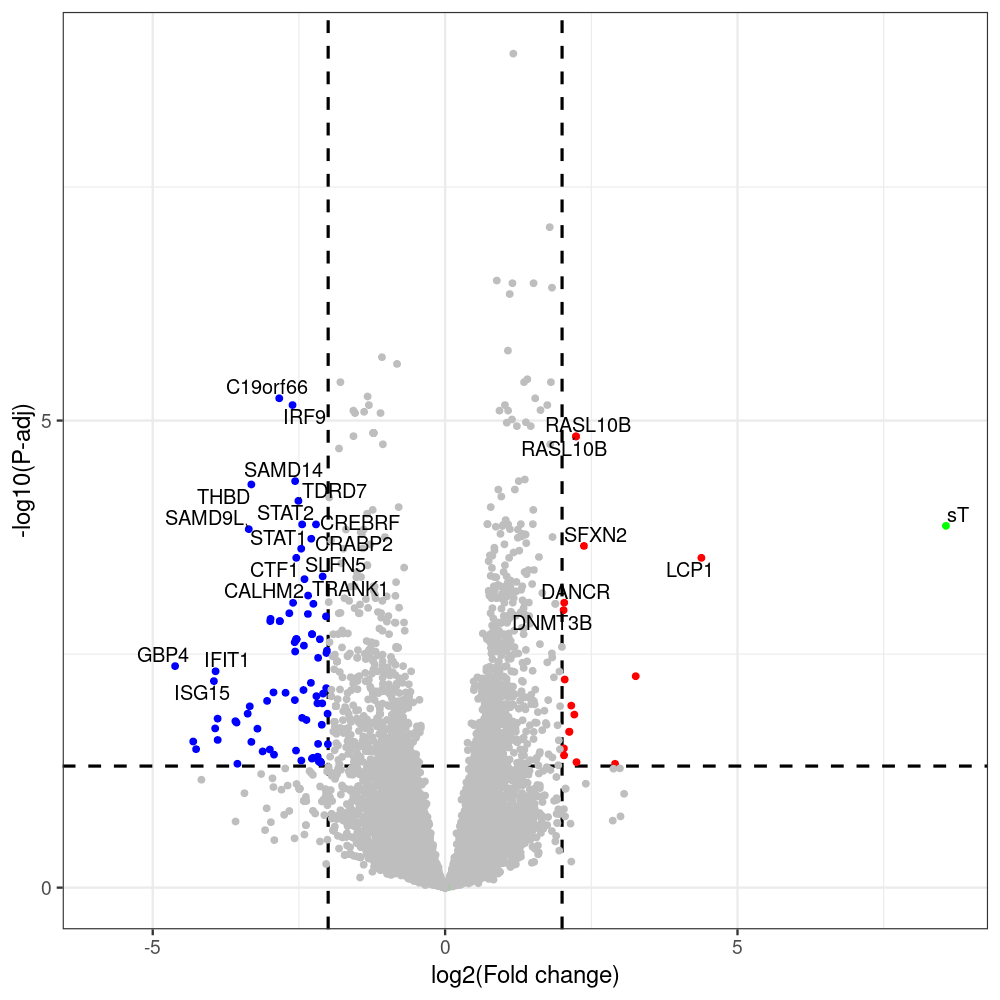
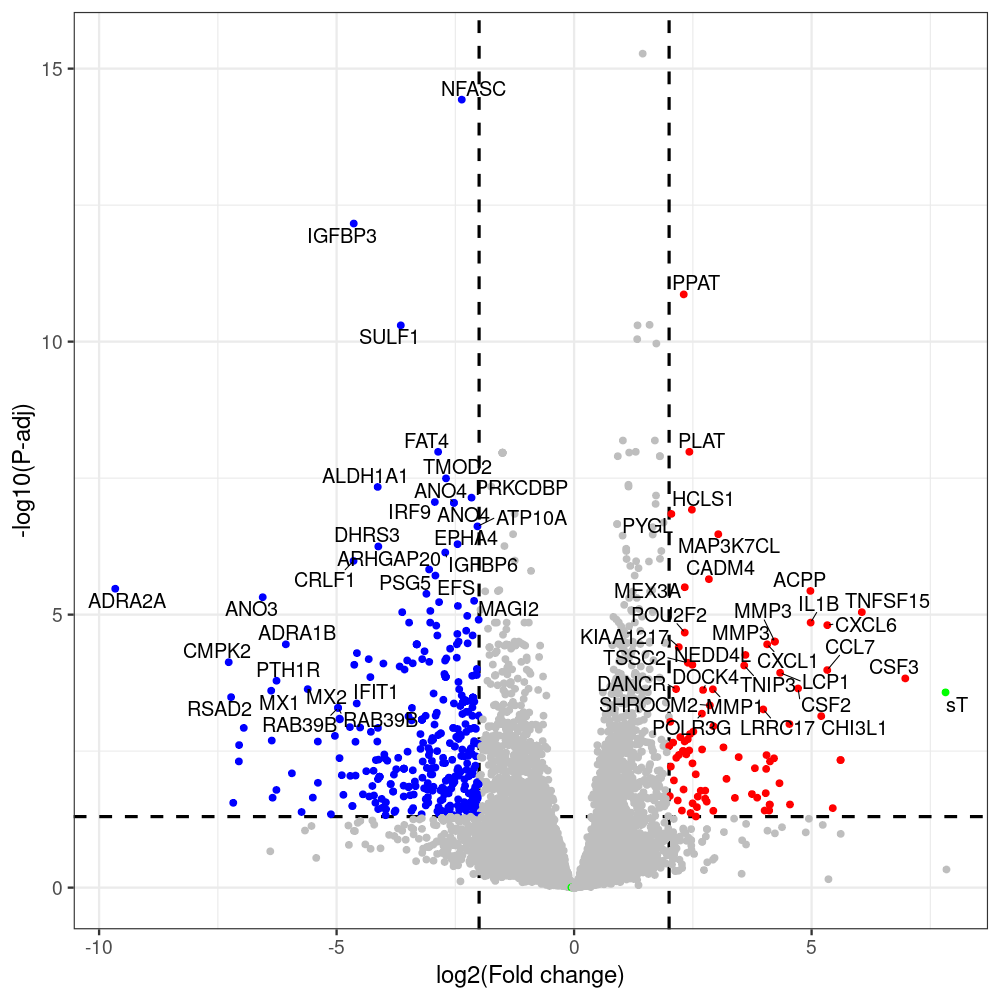
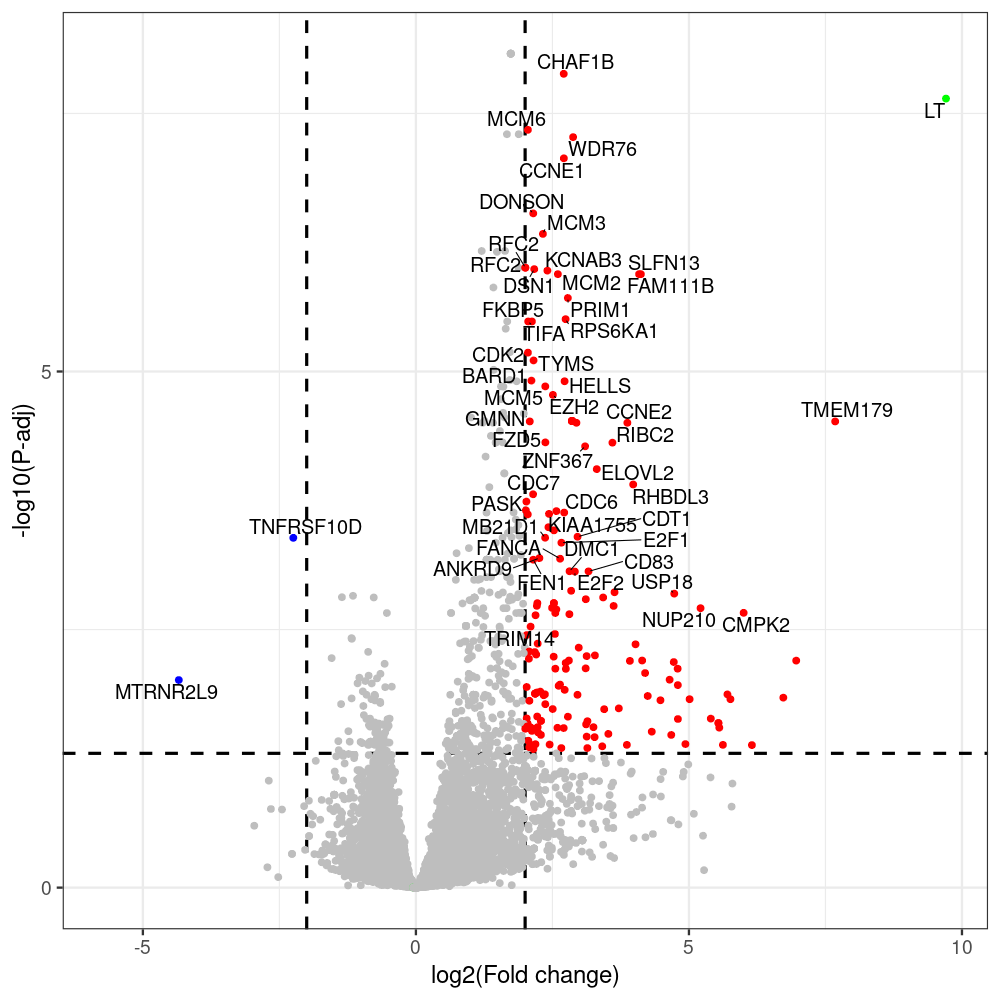
sT manuscript revision: log2FC of sT itself at 3 and 8 dpt
gene_x 0 like s 1597 view s
Tags: plot, R, research
-
nextflow
#under hamm ln -s /home/jhuang/Tools/nf-core-rnaseq-3.12.0/ rnaseq #Debug the following error: added "--minAssignedFrags 0 \\" to modules/nf-core/salmon/quant/main.nf option "salmon quant" and added "--min_mapped_reads 0" in the nextflow command (rnaseq_2021) jhuang@hamm:~/DATA/Data_Denise_sT_RNASeq$ /usr/local/bin/nextflow run rnaseq/main.nf --input samplesheet.csv --outdir results_JN707599 --fasta JN707599.fasta --gtf JN707599.gtf -profile test_full -resume --max_memory 124.GB --max_time 2400.h --save_reference --aligner star_salmon --gtf_extra_attributes gene_id --gtf_group_features transcript_id --featurecounts_group_type gene_id --featurecounts_feature_type transcript --skip_rseqc --skip_dupradar --skip_preseq --skip_biotype_qc --skip_deseq2_qc --skip_multiqc --min_mapped_reads 0 (rnaseq_2021) jhuang@hamm:~/DATA/Data_Denise_sT_RNASeq$ /usr/local/bin/nextflow run rnaseq/main.nf --input samplesheet.csv --outdir results_LT --fasta JN707599.fasta --gtf LT.gtf -profile test_full -resume --max_memory 124.GB --max_time 2400.h --save_reference --aligner star_salmon --gtf_extra_attributes gene_id --gtf_group_features transcript_id --featurecounts_group_type gene_id --featurecounts_feature_type transcript --skip_rseqc --skip_dupradar --skip_preseq --skip_biotype_qc --skip_deseq2_qc --skip_multiqc --min_mapped_reads 0 (rnaseq_2021) jhuang@hamm:~/DATA/Data_Denise_sT_RNASeq$ /usr/local/bin/nextflow run rnaseq/main.nf --input samplesheet.csv --outdir results_sT --fasta JN707599.fasta --gtf sT.gtf -profile test_full -resume --max_memory 124.GB --max_time 2400.h --save_reference --aligner star_salmon --gtf_extra_attributes gene_id --gtf_group_features transcript_id --featurecounts_group_type gene_id --featurecounts_feature_type transcript --skip_rseqc --skip_dupradar --skip_preseq --skip_biotype_qc --skip_deseq2_qc --skip_multiqc --min_mapped_reads 0 #DEBUG #V_8_4_1_p602_d8_DonorI,V_8_4_2_p602_d8_DonorI.fastq.gz,,auto #V_8_5_1_p602and604_d3_DonorI,V_8_5_1_602and604_d3_DonorI.fastq.gz,,auto #V_8_5_2_p602and604_d3_DonorII,V_8_5_2_602and604_d3_DonorII.fastq.gz,,auto #mamba install -c bioconda rsem #mamba install fq -
construct DESeqDataSet including host+viral gene expressions
# Import the required libraries library("AnnotationDbi") library("clusterProfiler") library("ReactomePA") library(gplots) library(tximport) library(DESeq2) library("org.Hs.eg.db") library(dplyr) library(tidyverse) setwd("~/DATA/Data_Denise_sT_immune_evasion_PUBLISHING/Data_Denise_RNASeq/results_JN707599/star_salmon") # Define paths to your Salmon output quantification files files <- c("V_8_0_mock_DonorI" = "./V_8_0_mock_DonorI/quant.sf", "V_8_0_mock_DonorII" = "./V_8_0_mock_DonorII/quant.sf", "V_8_1_5_p601_d3_DonorII" = "./V_8_1_5_p601_d3_DonorII/quant.sf", "V_8_1_5_p604_d3_DonorII" = "./V_8_1_5_p604_d3_DonorII/quant.sf", "V_8_1_5_p601_d8_DonorII" = "./V_8_1_5_p601_d8_DonorII/quant.sf", "V_8_1_5_p604_d8_DonorII" = "./V_8_1_5_p604_d8_DonorII/quant.sf", "V_8_1_6_p601_d3_DonorI" = "./V_8_1_6_p601_d3_DonorI/quant.sf", "V_8_1_6_p604_d3_DonorI" = "./V_8_1_6_p604_d3_DonorI/quant.sf", "V_8_1_6_p601_d8_DonorI" = "./V_8_1_6_p601_d8_DonorI/quant.sf", "V_8_1_6_p604_d8_DonorI" = "./V_8_1_6_p604_d8_DonorI/quant.sf", "V_8_2_3_p600_d3_DonorII" = "./V_8_2_3_p600_d3_DonorII/quant.sf", "V_8_2_3_p605_d3_DonorII" = "./V_8_2_3_p605_d3_DonorII/quant.sf", "V_8_2_3_p600_d8_DonorII" = "./V_8_2_3_p600_d8_DonorII/quant.sf", "V_8_2_3_p605_d8_DonorII" = "./V_8_2_3_p605_d8_DonorII/quant.sf", "V_8_2_4_p600_d3_DonorI" = "./V_8_2_4_p600_d3_DonorI/quant.sf", "V_8_2_4_p605_d3_DonorI" = "./V_8_2_4_p605_d3_DonorI/quant.sf", "V_8_2_4_p600_d8_DonorI" = "./V_8_2_4_p600_d8_DonorI/quant.sf", "V_8_2_4_p605_d8_DonorI" = "./V_8_2_4_p605_d8_DonorI/quant.sf", "V_8_4_1_p602_d8_DonorII" = "./V_8_4_1_p602_d8_DonorII/quant.sf", "V_8_4_1_p602_d8_DonorI" = "./V_8_4_1_p602_d8_DonorI/quant.sf", "V_8_3_1_p600and601_d12_DonorI" = "./V_8_3_1_p600and601_d12_DonorI/quant.sf", "V_8_3_1_p604and605_d12_DonorI" = "./V_8_3_1_p604and605_d12_DonorI/quant.sf", "V_8_3_2_p600and601_d9_DonorII" = "./V_8_3_2_p600and601_d9_DonorII/quant.sf", "V_8_3_2_p604and605_d9_DonorII" = "./V_8_3_2_p604and605_d9_DonorII/quant.sf", "V_8_4_2_p602_d3_DonorI" = "./V_8_4_2_p602_d3_DonorI/quant.sf", "V_8_4_2_p602_d3_DonorII" = "./V_8_4_2_p602_d3_DonorII/quant.sf", "V_8_5_1_p602and604_d3_DonorI" = "./V_8_5_1_sTplusLT_d3_Donor1/quant.sf", "V_8_5_2_p602and604_d3_DonorII" = "./V_8_5_2_sTplusLT_d3_Donor2/quant.sf") # Import the transcript abundance data with tximport txi <- tximport(files, type = "salmon", txIn = TRUE, txOut = TRUE) column_names <- colnames(txi$counts) output_string <- paste(column_names, collapse = ", ") cat(output_string, "\n") #"V_8_0_mock_DonorI","V_8_0_mock_DonorII","V_8_1_5_p601_d3_DonorII","V_8_1_5_p604_d3_DonorII","V_8_1_5_p601_d8_DonorII","V_8_1_5_p604_d8_DonorII","V_8_1_6_p601_d3_DonorI","V_8_1_6_p604_d3_DonorI","V_8_1_6_p601_d8_DonorI","V_8_1_6_p604_d8_DonorI","V_8_2_3_p600_d3_DonorII","V_8_2_3_p605_d3_DonorII","V_8_2_3_p600_d8_DonorII","V_8_2_3_p605_d8_DonorII","V_8_2_4_p600_d3_DonorI","V_8_2_4_p605_d3_DonorI","V_8_2_4_p600_d8_DonorI","V_8_2_4_p605_d8_DonorI","V_8_4_1_p602_d8_DonorII","V_8_4_1_p602_d8_DonorI","V_8_3_1_p600and601_d12_DonorI","V_8_3_1_p604and605_d12_DonorI","V_8_3_2_p600and601_d9_DonorII","V_8_3_2_p604and605_d9_DonorII","V_8_4_2_p602_d3_DonorI","V_8_4_2_p602_d3_DonorII","V_8_5_1_p602and604_d3_DonorI","V_8_5_2_p602and604_d3_DonorII" col_order <- c("V_8_0_mock_DonorI","V_8_0_mock_DonorII","V_8_1_5_p601_d3_DonorII","V_8_1_5_p604_d3_DonorII","V_8_1_5_p601_d8_DonorII","V_8_1_5_p604_d8_DonorII","V_8_1_6_p601_d3_DonorI","V_8_1_6_p604_d3_DonorI","V_8_1_6_p601_d8_DonorI","V_8_1_6_p604_d8_DonorI","V_8_2_3_p600_d3_DonorII","V_8_2_3_p605_d3_DonorII","V_8_2_3_p600_d8_DonorII","V_8_2_3_p605_d8_DonorII","V_8_2_4_p600_d3_DonorI","V_8_2_4_p605_d3_DonorI","V_8_2_4_p600_d8_DonorI","V_8_2_4_p605_d8_DonorI","V_8_4_1_p602_d8_DonorII","V_8_4_1_p602_d8_DonorI","V_8_3_1_p600and601_d12_DonorI","V_8_3_1_p604and605_d12_DonorI","V_8_3_2_p600and601_d9_DonorII","V_8_3_2_p604and605_d9_DonorII","V_8_4_2_p602_d3_DonorI","V_8_4_2_p602_d3_DonorII","V_8_5_1_p602and604_d3_DonorI","V_8_5_2_p602and604_d3_DonorII") #reordered.txi <- txi[,col_order] identical(column_names,col_order) condition = as.factor(c("untreated","untreated", "p601_d3","p604_d3", "p601_d8","p604_d8", "p601_d3","p604_d3","p601_d8","p604_d8", "p600_d3","p605_d3","p600_d8", "p605_d8", "p600_d3","p605_d3","p600_d8","p605_d8", "p602_d8","p602_d8", "p600and601_d9d12", "p604and605_d9d12","p600and601_d9d12","p604and605_d9d12", "p602_d3","p602_d3", "p602and604_d3","p602and604_d3")) batch = as.factor(c("200420", "200420", "190927", "190927", "190927", "190927", "190228", "190228", "190228", "190228", "191008", "191008", "191008", "191008", "190228", "190228", "190228", "190228", "200817", "200817", "200420", "200420", "200817", "200817", "210302", "210302", "210504","210504")) ids = as.factor(c("untreated_DonorI","untreated_DonorII", "p601_d3_DonorII","p604_d3_DonorII", "p601_d8_DonorII","p604_d8_DonorII", "p601_d3_DonorI","p604_d3_DonorI","p601_d8_DonorI","p604_d8_DonorI", "p600_d3_DonorII","p605_d3_DonorII","p600_d8_DonorII", "p605_d8_DonorII", "p600_d3_DonorI","p605_d3_DonorI","p600_d8_DonorI","p605_d8_DonorI", "p602_d8_DonorII","p602_d8_DonorI", "p600and601_d12_DonorI", "p604and605_d12_DonorI","p600and601_d9_DonorII","p604and605_d9_DonorII", "p602_d3_DonorI","p602_d3_DonorII", "p602and604_d3_DonorI","p602and604_d3_DonorII")) donor = as.factor(c("DonorI","DonorII", "DonorII","DonorII", "DonorII","DonorII", "DonorI","DonorI","DonorI","DonorI", "DonorII","DonorII","DonorII","DonorII", "DonorI","DonorI","DonorI","DonorI", "DonorII","DonorI", "DonorI", "DonorI","DonorII","DonorII", "DonorI","DonorII", "DonorI","DonorII")) # Define the colData for DESeq2 #colData = data.frame(row.names=column_names, condition=condition, donor=donor, batch=batch, ids=ids) colData <- data.frame(condition=condition, donor=donor, row.names=names(files)) # -- transcript-level count data (for virus) -- # Create DESeqDataSet object dds <- DESeqDataSetFromTximport(txi, colData=colData, design=~condition+donor) write.csv(counts(dds), file="transcript_counts.csv") # -- gene-level count data (for virus) -- # Read in the tx2gene map from salmon_tx2gene.tsv tx2gene <- read.table("salmon_tx2gene.tsv", header=FALSE, stringsAsFactors=FALSE) # Set the column names colnames(tx2gene) <- c("transcript_id", "gene_id", "gene_name") # Remove the gene_name column if not needed tx2gene <- tx2gene[,1:2] # Import and summarize the Salmon data with tximport txi <- tximport(files, type = "salmon", tx2gene = tx2gene, txOut = FALSE) dds <- DESeqDataSetFromTximport(txi, colData=colData, design=~condition+donor) #dds <- dds[rowSums(counts(dds) > 3) > 2, ] #60605-->26543 write.csv(counts(dds, normalized=FALSE), file="gene_counts.csv") # -- merge the raw counts of human and microbe -- setwd("~/DATA/Data_Denise_sT_immune_evasion_PUBLISHING/Data_Denise_RNASeq/results/featureCounts") d.raw<- read.delim2("merged_gene_counts_2_f3_f5.txt",sep="\t", header=TRUE, row.names=1) colnames(d.raw)<- c("V_8_0_mock_DonorI","V_8_1_6_p601_d8_DonorI","V_8_1_5_p604_d3_DonorII","V_8_1_6_p604_d3_DonorI","V_8_2_3_p605_d8_DonorII","V_8_0_mock_DonorII","V_8_1_5_p601_d8_DonorII","V_8_2_3_p605_d3_DonorII","V_8_1_6_p604_d8_DonorI","V_8_2_3_p600_d8_DonorII","V_8_2_4_p600_d8_DonorI","V_8_2_4_p600_d3_DonorI","V_8_1_5_p604_d8_DonorII","V_8_1_5_p601_d3_DonorII","V_8_1_6_p601_d3_DonorI","V_8_2_3_p600_d3_DonorII","V_8_2_4_p605_d3_DonorI","V_8_4_1_p602_d8_DonorI","V_8_3_2_p604and605_d9_DonorII","V_8_4_2_p602_d3_DonorI","V_8_4_2_p602_d3_DonorII","V_8_2_4_p605_d8_DonorI","V_8_3_1_p600and601_d12_DonorI","V_8_4_1_p602_d8_DonorII","V_8_3_1_p604and605_d12_DonorI","V_8_3_2_p600and601_d9_DonorII", "V_8_5_1_p602and604_d3_DonorI", "V_8_5_2_p602and604_d3_DonorII") #26364 col_order <- c("V_8_0_mock_DonorI","V_8_0_mock_DonorII","V_8_1_5_p601_d3_DonorII", "V_8_1_5_p604_d3_DonorII", "V_8_1_5_p601_d8_DonorII","V_8_1_5_p604_d8_DonorII", "V_8_1_6_p601_d3_DonorI","V_8_1_6_p604_d3_DonorI","V_8_1_6_p601_d8_DonorI","V_8_1_6_p604_d8_DonorI", "V_8_2_3_p600_d3_DonorII","V_8_2_3_p605_d3_DonorII","V_8_2_3_p600_d8_DonorII", "V_8_2_3_p605_d8_DonorII", "V_8_2_4_p600_d3_DonorI","V_8_2_4_p605_d3_DonorI","V_8_2_4_p600_d8_DonorI","V_8_2_4_p605_d8_DonorI", "V_8_4_1_p602_d8_DonorII","V_8_4_1_p602_d8_DonorI", "V_8_3_1_p600and601_d12_DonorI", "V_8_3_1_p604and605_d12_DonorI","V_8_3_2_p600and601_d9_DonorII","V_8_3_2_p604and605_d9_DonorII", "V_8_4_2_p602_d3_DonorI","V_8_4_2_p602_d3_DonorII", "V_8_5_1_p602and604_d3_DonorI", "V_8_5_2_p602and604_d3_DonorII") reordered.raw <- d.raw[,col_order] write.csv(reordered.raw, file="merged_gene_counts_2_f3_f5_reordered.txt") # Confirm the identification of the headers of two files before merging! #kate ./results/featureCounts/merged_gene_counts_2_f3_f5_reordered.txt #"","V_8_0_mock_DonorI","V_8_0_mock_DonorII","V_8_1_5_p601_d3_DonorII","V_8_1_5_p604_d3_DonorII","V_8_1_5_p601_d8_DonorII","V_8_1_5_p604_d8_DonorII","V_8_1_6_p601_d3_DonorI","V_8_1_6_p604_d3_DonorI","V_8_1_6_p601_d8_DonorI","V_8_1_6_p604_d8_DonorI","V_8_2_3_p600_d3_DonorII","V_8_2_3_p605_d3_DonorII","V_8_2_3_p600_d8_DonorII","V_8_2_3_p605_d8_DonorII","V_8_2_4_p600_d3_DonorI","V_8_2_4_p605_d3_DonorI","V_8_2_4_p600_d8_DonorI","V_8_2_4_p605_d8_DonorI","V_8_4_1_p602_d8_DonorII","V_8_4_1_p602_d8_DonorI","V_8_3_1_p600and601_d12_DonorI","V_8_3_1_p604and605_d12_DonorI","V_8_3_2_p600and601_d9_DonorII","V_8_3_2_p604and605_d9_DonorII","V_8_4_2_p602_d3_DonorI","V_8_4_2_p602_d3_DonorII","V_8_5_1_p602and604_d3_DonorI","V_8_5_2_p602and604_d3_DonorII" #results_JN707599/star_salmon/gene_counts.csv #"","V_8_0_mock_DonorI","V_8_0_mock_DonorII","V_8_1_5_p601_d3_DonorII","V_8_1_5_p604_d3_DonorII","V_8_1_5_p601_d8_DonorII","V_8_1_5_p604_d8_DonorII","V_8_1_6_p601_d3_DonorI","V_8_1_6_p604_d3_DonorI","V_8_1_6_p601_d8_DonorI","V_8_1_6_p604_d8_DonorI","V_8_2_3_p600_d3_DonorII","V_8_2_3_p605_d3_DonorII","V_8_2_3_p600_d8_DonorII","V_8_2_3_p605_d8_DonorII","V_8_2_4_p600_d3_DonorI","V_8_2_4_p605_d3_DonorI","V_8_2_4_p600_d8_DonorI","V_8_2_4_p605_d8_DonorI","V_8_4_1_p602_d8_DonorII","V_8_4_1_p602_d8_DonorI","V_8_3_1_p600and601_d12_DonorI","V_8_3_1_p604and605_d12_DonorI","V_8_3_2_p600and601_d9_DonorII","V_8_3_2_p604and605_d9_DonorII","V_8_4_2_p602_d3_DonorI","V_8_4_2_p602_d3_DonorII","V_8_5_1_p602and604_d3_DonorI","V_8_5_2_p602and604_d3_DonorII" #cat ../Data_Denise_RNASeq/results/featureCounts/merged_gene_counts_2_f3_f5_reordered.txt ../Data_Denise_RNASeq/results_JN707599/star_salmon/gene_counts.csv > merged_gene_counts.csv #~/Tools/csv2xls-0.4/csv_to_xls.py merged_gene_counts.csv -d',' -o raw_gene_counts.xls; setwd("~/DATA/Data_Denise_sT_immune_evasion_PUBLISHING/Data_Denise_RNASeq_Merged/") d.raw <- read.csv("merged_gene_counts.csv", header=TRUE, row.names=1) dds <- DESeqDataSetFromMatrix(countData=d.raw, colData=colData, design=~condition+donor) dim(counts(dds)) head(counts(dds), 10) rld <- rlogTransformation(dds) # draw simple pca and heatmap library(gplots) library("RColorBrewer") #mat <- assay(rld) #mm <- model.matrix(~condition, colData(rld)) #mat <- limma::removeBatchEffect(mat, batch=rld$batch, design=mm) #assay(rld) <- mat # -- pca -- png("pca.png", 1200, 800) plotPCA(rld, intgroup=c("condition")) dev.off() # -- heatmap -- png("heatmap.png", 1200, 800) distsRL <- dist(t(assay(rld))) mat <- as.matrix(distsRL) hc <- hclust(distsRL) hmcol <- colorRampPalette(brewer.pal(9,"GnBu"))(100) heatmap.2(mat, Rowv=as.dendrogram(hc),symm=TRUE, trace="none",col = rev(hmcol), margin=c(13, 13)) dev.off() -
(corrected of last step) construct DESeqDataSet including host+viral gene expressions
column_names <- colnames(d.raw) col_order <- c("V_8_0_mock_DonorI","V_8_0_mock_DonorII","V_8_1_5_p601_d3_DonorII","V_8_1_5_p604_d3_DonorII","V_8_1_5_p601_d8_DonorII","V_8_1_5_p604_d8_DonorII","V_8_1_6_p601_d3_DonorI","V_8_1_6_p604_d3_DonorI","V_8_1_6_p601_d8_DonorI","V_8_1_6_p604_d8_DonorI","V_8_2_3_p600_d3_DonorII","V_8_2_3_p605_d3_DonorII","V_8_2_3_p600_d8_DonorII","V_8_2_3_p605_d8_DonorII","V_8_2_4_p600_d3_DonorI","V_8_2_4_p605_d3_DonorI","V_8_2_4_p600_d8_DonorI","V_8_2_4_p605_d8_DonorI","V_8_4_1_p602_d8_DonorII","V_8_4_1_p602_d8_DonorI","V_8_3_1_p600and601_d12_DonorI","V_8_3_1_p604and605_d12_DonorI","V_8_3_2_p600and601_d9_DonorII","V_8_3_2_p604and605_d9_DonorII","V_8_4_2_p602_d3_DonorI","V_8_4_2_p602_d3_DonorII","V_8_5_1_p602and604_d3_DonorI","V_8_5_2_p602and604_d3_DonorII") identical(column_names,col_order) condition = as.factor(c("untreated","untreated", "p601_d3","p604_d3", "p601_d8","p604_d8", "p601_d3","p604_d3","p601_d8","p604_d8", "p600_d3","p605_d3","p600_d8", "p605_d8", "p600_d3","p605_d3","p600_d8","p605_d8", "p602_d8","p602_d8", "p600and601_d9d12", "p604and605_d9d12","p600and601_d9d12","p604and605_d9d12", "p602_d3","p602_d3", "p602and604_d3","p602and604_d3")) batch = as.factor(c("200420", "200420", "190927", "190927", "190927", "190927", "190228", "190228", "190228", "190228", "191008", "191008", "191008", "191008", "190228", "190228", "190228", "190228", "200817", "200817", "200420", "200420", "200817", "200817", "210302", "210302", "210504","210504")) ids = as.factor(c("untreated_DonorI","untreated_DonorII", "p601_d3_DonorII","p604_d3_DonorII", "p601_d8_DonorII","p604_d8_DonorII", "p601_d3_DonorI","p604_d3_DonorI","p601_d8_DonorI","p604_d8_DonorI", "p600_d3_DonorII","p605_d3_DonorII","p600_d8_DonorII", "p605_d8_DonorII", "p600_d3_DonorI","p605_d3_DonorI","p600_d8_DonorI","p605_d8_DonorI", "p602_d8_DonorII","p602_d8_DonorI", "p600and601_d12_DonorI", "p604and605_d12_DonorI","p600and601_d9_DonorII","p604and605_d9_DonorII", "p602_d3_DonorI","p602_d3_DonorII", "p602and604_d3_DonorI","p602and604_d3_DonorII")) donor = as.factor(c("DonorI","DonorII", "DonorII","DonorII", "DonorII","DonorII", "DonorI","DonorI","DonorI","DonorI", "DonorII","DonorII","DonorII","DonorII", "DonorI","DonorI","DonorI","DonorI", "DonorII","DonorI", "DonorI", "DonorI","DonorII","DonorII", "DonorI","DonorII", "DonorI","DonorII")) colData <- data.frame(condition=condition, donor=donor, row.names=column_names) setwd("~/DATA/Data_Denise_sT_PUBLISHING/Data_Denise_RNASeq_Merged_Corrected/") d.raw <- read.csv("merged_gene_counts_corrected.csv", header=TRUE, row.names=1) dds <- DESeqDataSetFromMatrix(countData=d.raw, colData=colData, design=~condition+donor) dim(counts(dds)) head(counts(dds), 10) rld <- rlogTransformation(dds) # draw simple pca and heatmap library(gplots) library("RColorBrewer") #mat <- assay(rld) #mm <- model.matrix(~condition, colData(rld)) #mat <- limma::removeBatchEffect(mat, batch=rld$batch, design=mm) #assay(rld) <- mat # -- pca -- png("pca.png", 1200, 800) plotPCA(rld, intgroup=c("condition")) dev.off() # -- heatmap -- png("heatmap.png", 1200, 800) distsRL <- dist(t(assay(rld))) mat <- as.matrix(distsRL) hc <- hclust(distsRL) hmcol <- colorRampPalette(brewer.pal(9,"GnBu"))(100) heatmap.2(mat, Rowv=as.dendrogram(hc),symm=TRUE, trace="none",col = rev(hmcol), margin=c(13, 13)) dev.off() -
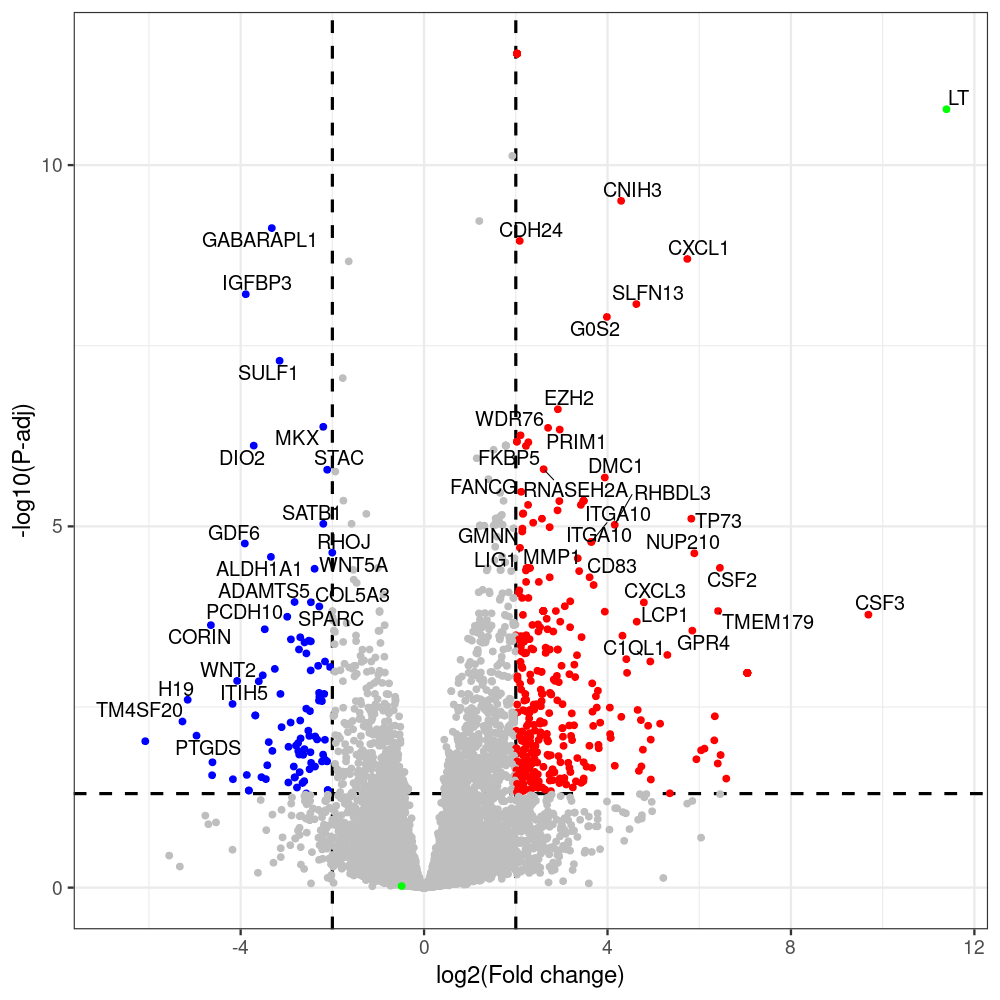
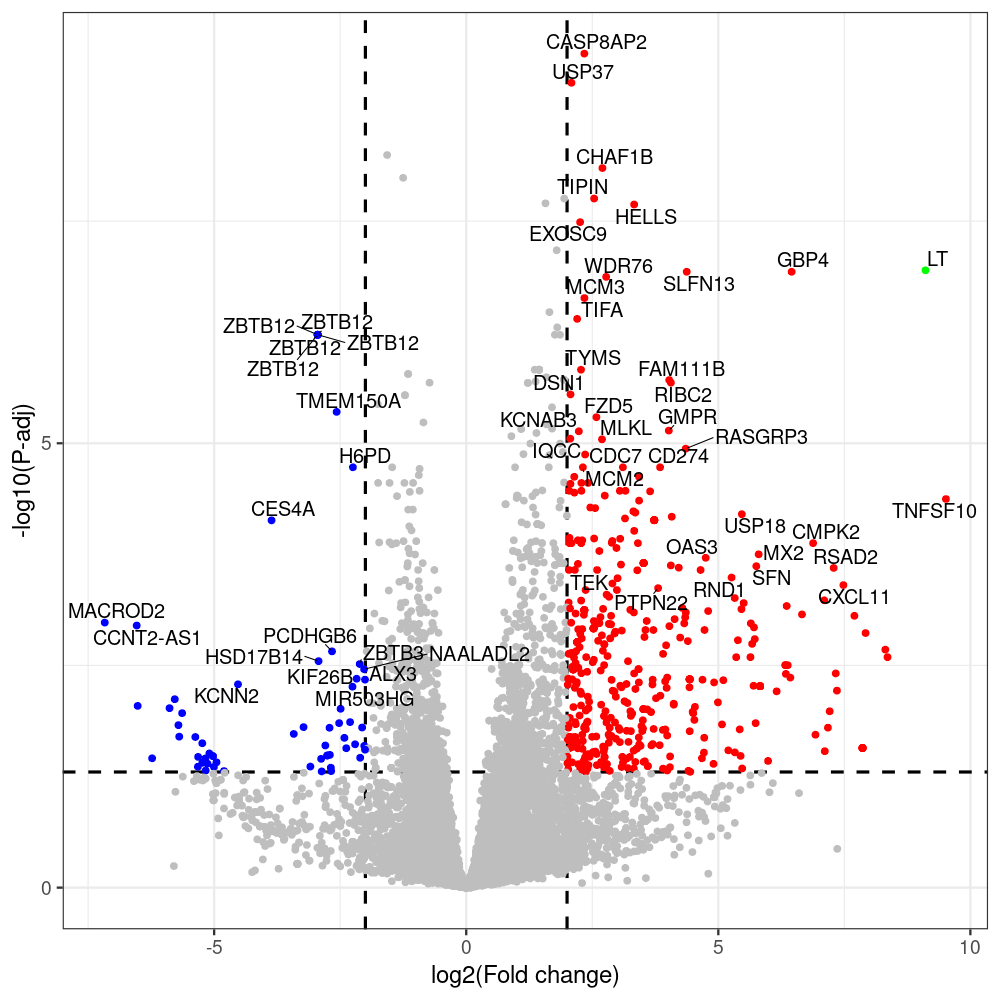
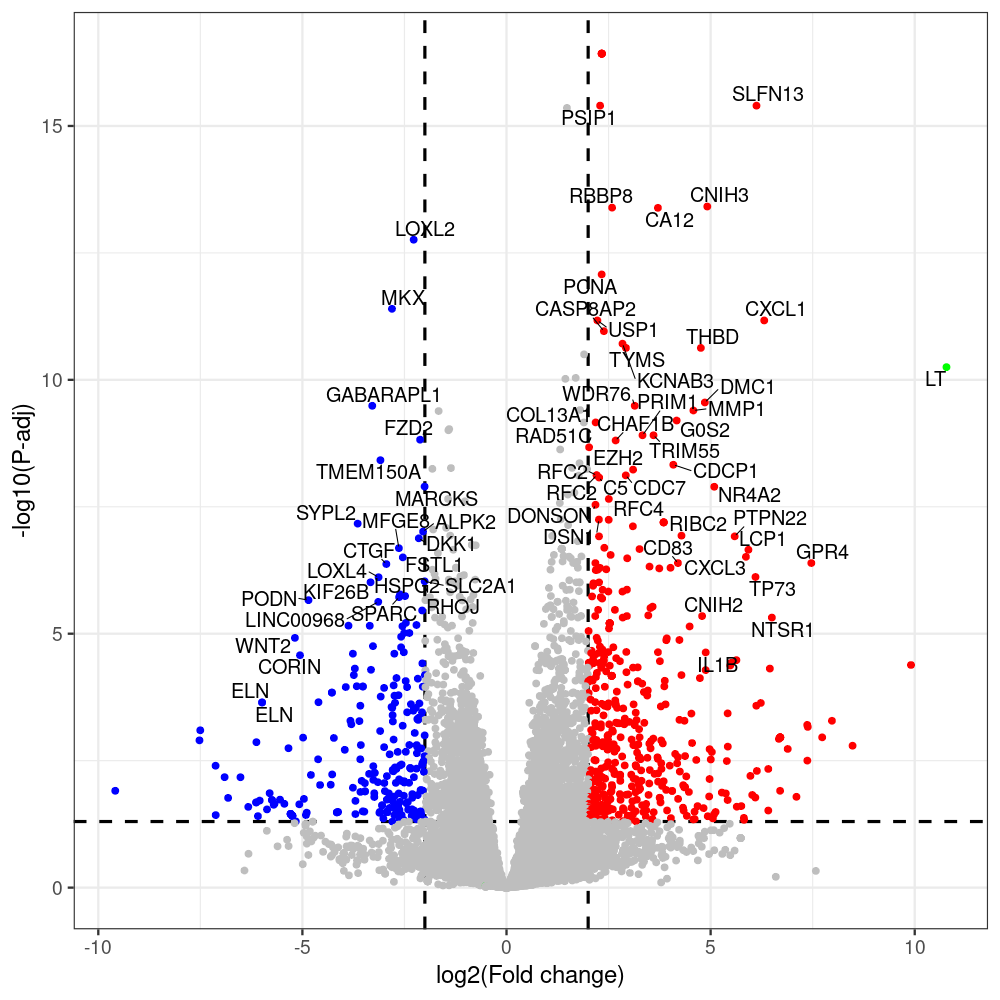
select the differentially expressed genes
#untreated_DonorI,untreated #untreated_DonorII,untreated #p601_d3_DonorII,mCherry control #p604_d3_DonorII,sT #p601_d8_DonorII,mCherry control #p604_d8_DonorII,sT #p601_d3_DonorI,mCherry control #p604_d3_DonorI,sT #p601_d8_DonorI,mCherry control #p604_d8_DonorI,sT # #p600_d3_DonorII,GFP control #p605_d3_DonorII,LTtr #p600_d8_DonorII,GFP control #p605_d8_DonorII,LTtr #p600_d3_DonorI,GFP control #p605_d3_DonorI,LTtr #p600_d8_DonorI,GFP control #p605_d8_DonorI,LTtr #p602_d3_DonorI,LT #p602_d3_DonorII,LT #p602_d8_DonorII,LT #p602_d8_DonorI,LT # #p600and601_d12_DonorI,GFP+mCherry control #p604and605_d12_DonorI,sT+LTtr #p600and601_d9_DonorII,GFP+mCherry control #p604and605_d9_DonorII,sT+LTtr # #p602and604_d3_DonorI,sT+LT #p602and604_d3_DonorII,sT+LT #CONSOLE: mkdir /home/jhuang/DATA/Data_Denise_sT_immune_evasion_PUBLISHING/Data_Denise_RNASeq_Merged/degenes #---- relevel to control ---- dds$condition <- relevel(dds$condition, "p601_d3") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("p604_d3_vs_p601_d3") dds$condition <- relevel(dds$condition, "p600_d3") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("p605_d3_vs_p600_d3") dds$condition <- relevel(dds$condition, "p600_d3") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("p602_d3_vs_p600_d3") dds$condition <- relevel(dds$condition, "p601_d8") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("p604_d8_vs_p601_d8") dds$condition <- relevel(dds$condition, "p600_d8") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("p605_d8_vs_p600_d8") dds$condition <- relevel(dds$condition, "p600_d8") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("p602_d8_vs_p600_d8") library(biomaRt) listEnsembl() listMarts() #--> total 69, 27 GRCh38.p7 and 39 GRCm38.p4 #ensembl <- useEnsembl(biomart = "ensembl", dataset = "hsapiens_gene_ensembl", version="104") #GRCh37.p13 ensembl <- useEnsembl("ensembl","hsapiens_gene_ensembl", host="https://grch37.ensembl.org") datasets <- listDatasets(ensembl) # -- 1. export res_df containing both human and virus genes -- #for (i in clist) { i<-clist[1] contrast = paste("condition", i, sep="_") res = results(dds, name=contrast) res <- res[!is.na(res$log2FoldChange),] res_df <- as.data.frame(res) write.csv(as.data.frame(res_df[order(res_df$pvalue),]), file = paste(i, "all.txt", sep="-")) up <- subset(res_df, padj<=0.05 & log2FoldChange>=2) down <- subset(res_df, padj<=0.05 & log2FoldChange<=-2) write.csv(as.data.frame(up[order(up$log2FoldChange,decreasing=TRUE),]), file = paste(i, "up.txt", sep="-")) write.csv(as.data.frame(down[order(abs(down$log2FoldChange),decreasing=TRUE),]), file = paste(i, "down.txt", sep="-")) #} # -- 2. annatete human genes 'geness_res', note that the virus genes in that is ignored in the process since they are not in the database -- #> dim(geness) [1] 21938 9 using GRCh38.p7 #> dim(geness) [1] 23517 9 using GRCh37.p13 #for (i in clist) { #i<-clist[1] contrast = paste("condition", i, sep="_") res = results(dds, name=contrast) res <- res[!is.na(res$log2FoldChange),] geness <- getBM(attributes = c('ensembl_gene_id', 'external_gene_name', 'gene_biotype', 'entrezgene_id', 'chromosome_name', 'start_position', 'end_position', 'strand', 'description'), filters = 'external_gene_name', values = rownames(res), mart = ensembl) geness_uniq <- distinct(geness, ensembl_gene_id, .keep_all= TRUE) #merge by column by common colunmn name, in the case "GENEID" res$geneSymbol = rownames(res) #identical(rownames(res), rownames(geness_uniq)) res_df <- as.data.frame(res) geness_res <- merge(geness_uniq, res_df, by.x="external_gene_name", by.y="geneSymbol") dim(geness_res) #22044 15 rownames(geness_res) <- geness_res$ensembl_gene_id geness_res$ensembl_gene_id <- NULL #} # -- 3. prepare annatete virus genes -- virus_genes <- c("LT", "sT", "VP1", "VP2", "VP3") virus_rows <- res_df[rownames(res_df) %in% virus_genes, ] virus_rows$external_gene_name <- rownames(virus_rows) virus_rows$chromosome_name <- "JN707599" # Define default values based on data type default_values <- list( character = NULL, numeric = 0, integer = 0L, logical = FALSE ) # Ensure that virus_rows has the same columns as geness_res for (col in colnames(geness_res)) { if (!col %in% colnames(virus_rows)) { data_type <- class(geness_res[[col]])[1] default_value <- default_values[[data_type]] virus_rows[[col]] <- rep(default_value, nrow(virus_rows)) } } missing_cols <- setdiff(colnames(geness_res), colnames(virus_rows)) for (col in missing_cols) { virus_rows[[col]] <- NA # Or another default value as appropriate } # Reorder columns in virus_rows to match the order in geness_res virus_rows <- virus_rows[, colnames(geness_res), drop = FALSE] # -- 4. merge them together -- #for (i in clist) { merged_df <- rbind(geness_res, virus_rows) merged_df_sorted <- as.data.frame(merged_df[order(merged_df$padj),]) write.csv(merged_df_sorted, file = paste(i, "all_annotated.txt", sep="-")) up <- subset(merged_df_sorted, padj<=0.05 & log2FoldChange>=2) down <- subset(merged_df_sorted, padj<=0.05 & log2FoldChange<=-2) viral <- merged_df_sorted[merged_df_sorted$external_gene_name %in% c("LT", "sT", "VP1", "VP2", "VP3"), ] write.csv(as.data.frame(up[order(up$log2FoldChange,decreasing=TRUE),]), file = paste(i, "up_annotated.txt", sep="-")) write.csv(as.data.frame(down[order(abs(down$log2FoldChange),decreasing=TRUE),]), file = paste(i, "down_annotated.txt", sep="-")) write.csv(as.data.frame(viral), file = paste(i, "viral_annotated.txt", sep="-")) #} # -- 5. draw graphics -- library(ggrepel) #geness_res <- read.csv(file = "p605_d8_vs_p600_d8-all_annotated.txt", sep=",", row.names=1) #i<-"p605_d8_vs_p600_d8" geness_res <- merged_df_sorted # Color setting geness_res$Color <- ifelse(geness_res$padj > 0.05 | abs(geness_res$log2FoldChange) < 2, "gray", ifelse(geness_res$log2FoldChange > 0, "red", "blue")) # Predefined genes colored in green predefined_genes <- c("LT", "sT", "VP1", "VP2", "VP3") geness_res$Color[geness_res$external_gene_name %in% predefined_genes] <- "green" geness_res$invert_Padj <- (-log10(geness_res$padj)) * sign(geness_res$log2FoldChange) top_g <- unique(c(geness_res[order(geness_res$invert_Padj, decreasing = TRUE), 'external_gene_name'][1:200], geness_res[order(geness_res$invert_Padj, decreasing = FALSE), 'external_gene_name'][1:200])) # Define the original and compressed ranges original_range <- c(80, 115) compressed_range <- c(80.0, 90.0) # Calculate breaks for the y-axis y_breaks_below <- seq(0, 80, by=5) y_breaks_compressed <- c(80.0, 90.0) y_breaks_above <- c() y_breaks <- c(y_breaks_below, y_breaks_compressed, y_breaks_above) y_labels_below <- seq(0, 80, by=5) y_labels_compressed <- c(80, 115) y_labels_above <- c() y_labels <- c(y_labels_below, y_labels_compressed, y_labels_above) # Adjust the p-values based on the ranges geness_res$adjusted_pvalue <- with(geness_res, ifelse(-log10(padj) > original_range[1] & -log10(padj) <= original_range[2], ((-log10(padj) - original_range[1]) / (original_range[2] - original_range[1])) * (compressed_range[2] - compressed_range[1]) + compressed_range[1], ifelse(-log10(padj) > original_range[2], -log10(padj) - (original_range[2] - original_range[1]) + (compressed_range[2] - compressed_range[1]), -log10(padj)))) # Create the plot png(paste(i, "png", sep="."), width=1000, height=1000) ggplot(geness_res, aes(x = log2FoldChange, y = adjusted_pvalue, color = Color, label = external_gene_name)) + geom_vline(xintercept = c(2, -2), lty = "dashed", size = 1.5) + geom_hline(yintercept = -log10(0.05), lty = "dashed", size = 1.5) + geom_point(size = 3) + labs(x = "log2(Fold change)", y = "-log10(P-adj)", color = "Significance") + scale_color_identity() + geom_text_repel(data = subset(geness_res, external_gene_name %in% top_g & padj < 0.05 & (abs(log2FoldChange) >= 2)), size = 7, point.padding = 0.15, color = "black", min.segment.length = .1, box.padding = .2, lwd = 2) + theme_bw(base_size = 24) + theme(legend.position = "bottom") + #annotate("rect", xmin = -Inf, xmax = Inf, ymin = compressed_range[1], ymax = compressed_range[1], linetype = "dashed", color = "grey") + #annotate("rect", xmin = -Inf, xmax = Inf, ymin = compressed_range[2], ymax = compressed_range[2], linetype = "dashed", color = "grey") + #annotate("text", x = -Inf, y = compressed_range[1], label = "/", hjust = 0, size = 10) + #annotate("text", x = -Inf, y = compressed_range[2], label = "/", hjust = 0, size = 10) + scale_y_continuous(breaks = sort(y_breaks), labels = sort(y_labels)) dev.off() #Under DIR degenes under KONSOLE and delete "start_position","end_position","strand" and so on. #~/Tools/csv2xls-0.4/csv_to_xls.py viral_gene_counts.csv p604_d8_vs_p601_d8-viral_annotated.txt p605_d8_vs_p600_d8-viral_annotated.txt p602_d8_vs_p600_d8-viral_annotated.txt p604_d3_vs_p601_d3-viral_annotated.txt p605_d3_vs_p600_d3-viral_annotated.txt p602_d3_vs_p600_d3-viral_annotated.txt -d$',' -o viral_raw_counts_and_comparisons.xls; #dpt: days post-transfection
点赞本文的读者
还没有人对此文章表态
本文有评论
nicknames of LT, LTtr, sT and so on说:
#untreated_DonorI,untreated #untreated_DonorII,untreated #p601_d3_DonorII,mCherry control #p604_d3_DonorII,sT #p601_d8_DonorII,mCherry control #p604_d8_DonorII,sT #p601_d3_DonorI,mCherry control #p604_d3_DonorI,sT #p601_d8_DonorI,mCherry control #p604_d8_DonorI,sT # #p600_d3_DonorII,GFP control #p605_d3_DonorII,LTtr #p600_d8_DonorII,GFP control #p605_d8_DonorII,LTtr #p600_d3_DonorI,GFP control #p605_d3_DonorI,LTtr #p600_d8_DonorI,GFP control #p605_d8_DonorI,LTtr #p602_d3_DonorI,LT #p602_d3_DonorII,LT #p602_d8_DonorII,LT #p602_d8_DonorI,LT # #p600and601_d12_DonorI,GFP+mCherry control #p604and605_d12_DonorI,sT+LTtr #p600and601_d9_DonorII,GFP+mCherry control #p604and605_d9_DonorII,sT+LTtr # #p602and604_d3_DonorI,sT+LT #p602and604_d3_DonorII,sT+LT
看文章,发评论,不要沉默
最受欢迎文章
- Motif Discovery in Biological Sequences: A Comparison of MEME and HOMER
- Calling peaks using findPeaks of HOMER
- Kraken2 Installation and Usage Guide
- Why Do Significant Gene Lists Change After Adding Additional Conditions in Differential Gene Expression Analysis?
- Should the inputs for GSVA be normalized or raw?
- PiCRUST2 Pipeline for Functional Prediction and Pathway Analysis in Metagenomics
- Updating Human Gene Identifiers using Ensembl BioMart: A Step-by-Step Guide
- pheatmap vs heatmap.2
- Setup conda environments
- Guide to Submitting Data to GEO (Gene Expression Omnibus)
最新文章
- Risks of Rebooting into Rescue Mode
- 足突(Podosome)、胞外囊泡(Extracellular Vesicle)与基质金属蛋白酶(MMPs)综合解析
- NCBI BioSample Submission Strategy for PJI and Nasal Microbiota Study
- Human RNA-seq processing for Data_Ben_RNAseq_2025
最多评论文章
- Updating Human Gene Identifiers using Ensembl BioMart: A Step-by-Step Guide
- The top 10 genes
- Retrieving KEGG Genes Using Bioservices in Python
推荐相似文章
MicrobiotaProcess Group2 vs Group6 (v1)
Bubble plot for 1457∆atlE vs 1457-M10 vs 1457 vs mock