Draw plots for piRNA generated by COMPSRA
gene_x 0 like s 889 view s
Tags: pipeline
-
Generate the following files according to STEPS 1-4 from http://xgenes.com/article/article-content/239/small-rna-sequencing-processing-in-the-example-of-smallrna-7/, http://xgenes.com/article/article-content/232/small-rna-sequencing-processing-in-the-example-of-smallrna-7/, and http://xgenes.com/article/article-content/156/small-rna-processing/. For COMPSRA_MERGE_0_miRNA.txt, we also need STEP 5 to add the read numbers of MCPyV-M1.
COMPSRA_MERGE_0_miRNA.txt COMPSRA_MERGE_0_piRNA.txt * COMPSRA_MERGE_0_snRNA.txt COMPSRA_MERGE_0_tRNA.txt COMPSRA_MERGE_0_snoRNA.txt COMPSRA_MERGE_0_circRNA.txt -
Input files for piRNA are two files: COMPSRA_MERGE_0_piRNA.txt and ids
-
COMPSRA_MERGE_0_piRNA.txt
#The former are more precise due to the reads from virus will be mapped on the virus-genome diff ./our_out_on_hg38+JN707599/COMPSRA_MERGE_0_piRNA.txt ./our_out_on_hg38/COMPSRA_MERGE_0_piRNA.txt diff ./our_out_on_hg38+JN707599/COMPSRA_MERGE_0_snRNA.txt ./our_out_on_hg38/COMPSRA_MERGE_0_snRNA.txt cp ../our_out_on_hg38+JN707599/COMPSRA_MERGE_0_piRNA.txt .
-
prepare the file ids
#see Option4: manully defining
-
-
Draw plots with R using DESeq2
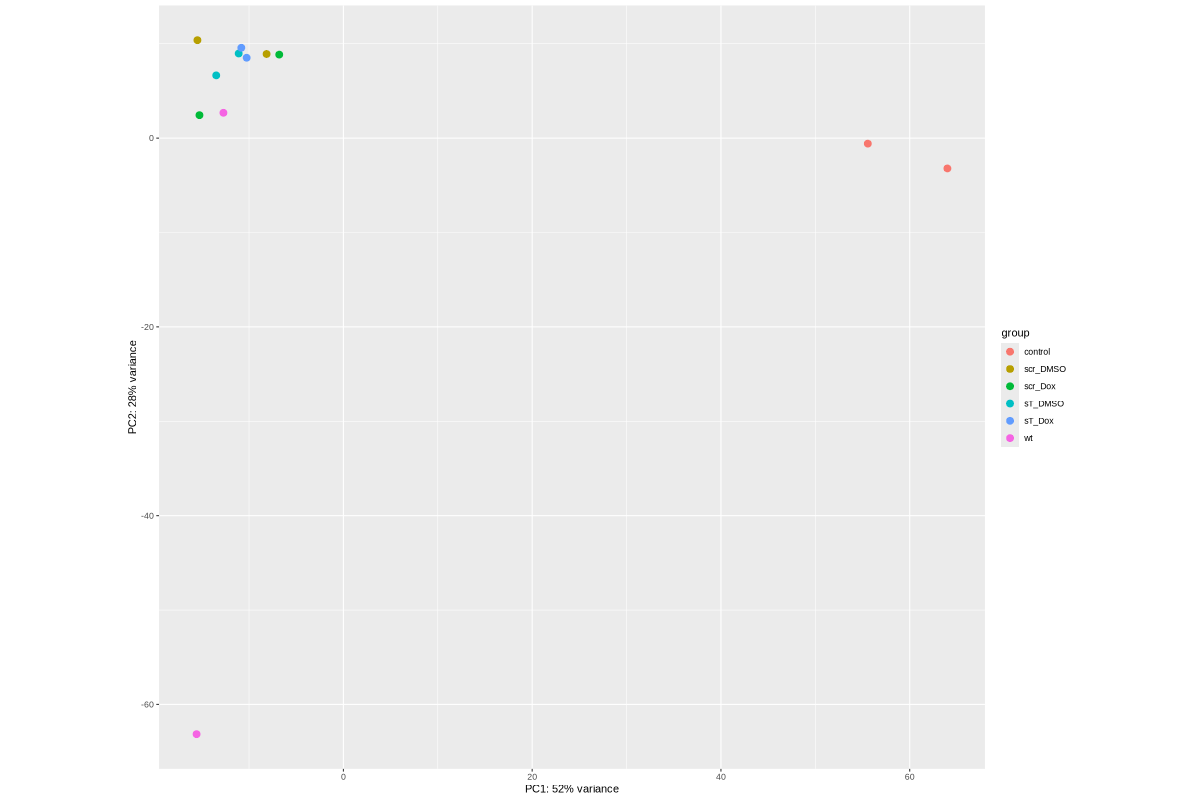
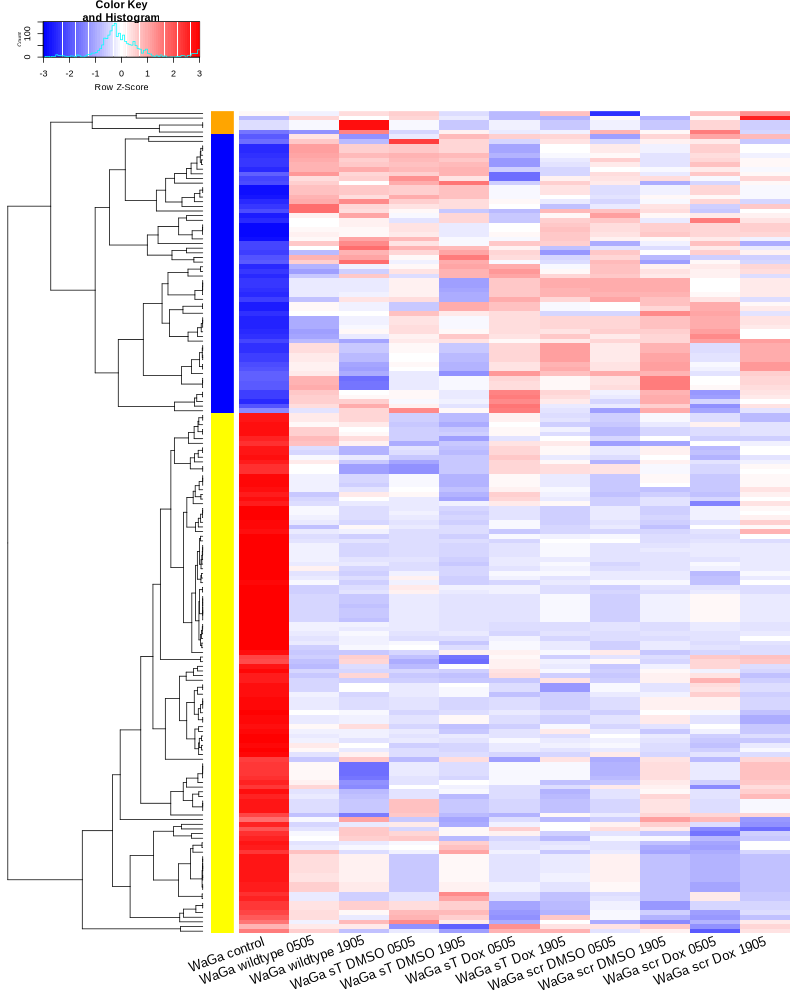
#BiocManager::install("AnnotationDbi") #BiocManager::install("clusterProfiler") #BiocManager::install(c("ReactomePA","org.Hs.eg.db")) #BiocManager::install("limma") library("AnnotationDbi") library("clusterProfiler") library("ReactomePA") library("org.Hs.eg.db") library(DESeq2) library(gplots) library(limma) # Check the current library paths .libPaths() #setwd("/home/jhuang/DATA/Data_Ute/Data_Ute_smallRNA_7/our_out_on_hg38+JN707599_2024_corrected/") d.raw<- read.delim2("COMPSRA_MERGE_0_piRNA.txt",sep="\t", header=TRUE, row.names=1) d.raw$X <- NULL d.raw[] <- lapply(d.raw, as.numeric) EV_or_parental = as.factor(c("EV","EV", "EV","EV", "EV","EV", "EV","EV", "EV","EV", "parental","parental")) donor = as.factor(c("0505","1905", "0505","1905", "0505","1905", "0505","1905", "0505","1905", "0505","1905")) replicates = as.factor(c("sT_DMSO","sT_DMSO", "sT_Dox","sT_Dox", "scr_DMSO","scr_DMSO", "scr_Dox","scr_Dox", "wt","wt", "control","control")) ids = as.factor(c("0505_WaGa_sT_DMSO","1905_WaGa_sT_DMSO","0505_WaGa_sT_Dox","1905_WaGa_sT_Dox","0505_WaGa_scr_DMSO","1905_WaGa_scr_DMSO","0505_WaGa_scr_Dox","1905_WaGa_scr_Dox","0505_WaGa_wt","1905_WaGa_wt","control_MKL1","control_WaGa")) cData = data.frame(row.names=colnames(d.raw), replicates=replicates, ids=ids, donor=donor, EV_or_parental=EV_or_parental) dds<-DESeqDataSetFromMatrix(countData=d.raw, colData=cData, design=~replicates+donor) rld <- rlogTransformation(dds) # -- before pca -- png("pca.png", 1200, 800) plotPCA(rld, intgroup=c("replicates")) #plotPCA(rld, intgroup = c("replicates", "batch")) #plotPCA(rld, intgroup = c("replicates", "ids")) #plotPCA(rld, "batch") dev.off() png("pca2.png", 1200, 800) plotPCA(rld, intgroup=c("donor")) dev.off() #### STEP2: DEGs #### #convert bam to bigwig using deepTools by feeding inverse of DESeq’s size Factor sizeFactors(dds) #NULL dds <- estimateSizeFactors(dds) sizeFactors(dds) normalized_counts <- counts(dds, normalized=TRUE) write.table(normalized_counts, file="normalized_counts.txt", sep="\t", quote=F, col.names=NA) #---- * to untreated ---- dds<-DESeqDataSetFromMatrix(countData=d.raw, colData=cData, design=~EV_or_parental+donor) dds$EV_or_parental <- relevel(dds$EV_or_parental, "parental") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("EV_vs_parental") for (i in clist) { contrast = paste("EV_or_parental", i, sep="_") res = results(dds, name=contrast) res <- res[!is.na(res$log2FoldChange),] #https://bioconductor.org/packages/release/bioc/vignettes/DESeq2/inst/doc/DESeq2.html#why-are-some-p-values-set-to-na res$padj <- ifelse(is.na(res$padj), 1, res$padj) res_df <- as.data.frame(res) write.csv(as.data.frame(res_df[order(res_df$pvalue),]), file = paste(i, "all.txt", sep="-")) up <- subset(res_df, padj<=0.1 & log2FoldChange>=2) down <- subset(res_df, padj<=0.1 & log2FoldChange<=-2) write.csv(as.data.frame(up[order(up$log2FoldChange,decreasing=TRUE),]), file = paste(i, "up.txt", sep="-")) write.csv(as.data.frame(down[order(abs(down$log2FoldChange),decreasing=TRUE),]), file = paste(i, "down.txt", sep="-")) } #~/Tools/csv2xls-0.4/csv_to_xls.py EV_vs_parental-all.txt EV_vs_parental-up.txt EV_vs_parental-down.txt -d$',' -o EV_vs_parental.xls; dds<-DESeqDataSetFromMatrix(countData=d.raw, colData=cData, design=~replicates+donor) dds$replicates <- relevel(dds$replicates, "sT_DMSO") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("sT_Dox_vs_sT_DMSO") dds$replicates <- relevel(dds$replicates, "scr_Dox") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("sT_Dox_vs_scr_Dox") dds$replicates <- relevel(dds$replicates, "scr_DMSO") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("scr_Dox_vs_scr_DMSO", "sT_DMSO_vs_scr_DMSO") for (i in clist) { contrast = paste("replicates", i, sep="_") res = results(dds, name=contrast) res <- res[!is.na(res$log2FoldChange),] #https://bioconductor.org/packages/release/bioc/vignettes/DESeq2/inst/doc/DESeq2.html#why-are-some-p-values-set-to-na res$padj <- ifelse(is.na(res$padj), 1, res$padj) res_df <- as.data.frame(res) write.csv(as.data.frame(res_df[order(res_df$pvalue),]), file = paste(i, "all.txt", sep="-")) up <- subset(res_df, padj<=0.1 & log2FoldChange>=2) down <- subset(res_df, padj<=0.1 & log2FoldChange<=-2) write.csv(as.data.frame(up[order(up$log2FoldChange,decreasing=TRUE),]), file = paste(i, "up.txt", sep="-")) write.csv(as.data.frame(down[order(abs(down$log2FoldChange),decreasing=TRUE),]), file = paste(i, "down.txt", sep="-")) } ~/Tools/csv2xls-0.4/csv_to_xls.py \ sT_Dox_vs_sT_DMSO-all.txt \ sT_Dox_vs_sT_DMSO-up.txt \ sT_Dox_vs_sT_DMSO-down.txt \ -d$',' -o sT_Dox_vs_sT_DMSO.xls; ~/Tools/csv2xls-0.4/csv_to_xls.py \ sT_Dox_vs_scr_Dox-all.txt \ sT_Dox_vs_scr_Dox-up.txt \ sT_Dox_vs_scr_Dox-down.txt \ -d$',' -o sT_Dox_vs_scr_Dox.xls; ~/Tools/csv2xls-0.4/csv_to_xls.py \ scr_Dox_vs_scr_DMSO-all.txt \ scr_Dox_vs_scr_DMSO-up.txt \ scr_Dox_vs_scr_DMSO-down.txt \ -d$',' -o scr_Dox_vs_scr_DMSO.xls; ~/Tools/csv2xls-0.4/csv_to_xls.py \ sT_DMSO_vs_scr_DMSO-all.txt \ sT_DMSO_vs_scr_DMSO-up.txt \ sT_DMSO_vs_scr_DMSO-down.txt \ -d$',' -o sT_DMSO_vs_scr_DMSO.xls; ##### STEP3: prepare all_genes ##### rld <- rlogTransformation(dds) mat <- assay(rld) mm <- model.matrix(~replicates, colData(rld)) mat <- limma::removeBatchEffect(mat, batch=rld$donor, design=mm) assay(rld) <- mat RNASeq.NoCellLine <- assay(rld) # reorder the columns colnames(RNASeq.NoCellLine) = c("0505 WaGa sT DMSO","1905 WaGa sT DMSO","0505 WaGa sT Dox","1905 WaGa sT Dox","0505 WaGa scr DMSO","1905 WaGa scr DMSO","0505 WaGa scr Dox","1905 WaGa scr Dox","0505 WaGa wt","1905 WaGa wt","control MKL1","control WaGa") col.order <-c("control MKL1", "control WaGa","0505 WaGa wt","1905 WaGa wt","0505 WaGa sT DMSO","1905 WaGa sT DMSO","0505 WaGa sT Dox","1905 WaGa sT Dox","0505 WaGa scr DMSO","1905 WaGa scr DMSO","0505 WaGa scr Dox","1905 WaGa scr Dox") RNASeq.NoCellLine <- RNASeq.NoCellLine[,col.order] #Option4: manully defining #for i in EV_vs_parental sT_Dox_vs_sT_DMSO sT_Dox_vs_scr_Dox scr_Dox_vs_scr_DMSO sT_DMSO_vs_scr_DMSO; do echo "cut -d',' -f1-1 ${i}-up.txt > ${i}-up.id"; echo "cut -d',' -f1-1 ${i}-down.txt > ${i}-down.id"; done #cat *.id | sort -u > ids ##add Gene_Id in the first line, delete the "" GOI <- read.csv("ids")$Gene_Id datamat = RNASeq.NoCellLine[GOI, ] ##### STEP4: clustering the genes and draw heatmap ##### datamat <- datamat[,-1] #delete the sample "control MKL1" colnames(datamat)[1] <- "WaGa control" #rename the isolate names according to the style of RNA-seq as follows? colnames(datamat)[2] <- "WaGa wildtype 0505" colnames(datamat)[3] <- "WaGa wildtype 1905" colnames(datamat)[4] <- "WaGa sT DMSO 0505" colnames(datamat)[5] <- "WaGa sT DMSO 1905" colnames(datamat)[6] <- "WaGa sT Dox 0505" colnames(datamat)[7] <- "WaGa sT Dox 1905" colnames(datamat)[8] <- "WaGa scr DMSO 0505" colnames(datamat)[9] <- "WaGa scr DMSO 1905" colnames(datamat)[10] <- "WaGa scr Dox 0505" colnames(datamat)[11] <- "WaGa scr Dox 1905" write.csv(datamat, file ="gene_expression_keeping_replicates.txt") #"ward.D"’, ‘"ward.D2"’,‘"single"’, ‘"complete"’, ‘"average"’ (= UPGMA), ‘"mcquitty"’(= WPGMA), ‘"median"’ (= WPGMC) or ‘"centroid"’ (= UPGMC) hr <- hclust(as.dist(1-cor(t(datamat), method="pearson")), method="complete") hc <- hclust(as.dist(1-cor(datamat, method="spearman")), method="complete") mycl = cutree(hr, h=max(hr$height)/1.5) mycol = c("YELLOW", "BLUE", "ORANGE", "CYAN", "GREEN", "MAGENTA", "GREY", "LIGHTCYAN", "RED", "PINK", "DARKORANGE", "MAROON", "LIGHTGREEN", "DARKBLUE", "DARKRED", "LIGHTBLUE", "DARKCYAN", "DARKGREEN", "DARKMAGENTA"); mycol = mycol[as.vector(mycl)] png("piRNA_heatmap_keeping_replicates.png", width=800, height=1000) #svg("DEGs_heatmap_keeping_replicates.svg", width=6, height=8) heatmap.2(as.matrix(datamat), Rowv=as.dendrogram(hr), Colv=NA, dendrogram='row', labRow="", scale='row', trace='none', col=bluered(75), RowSideColors=mycol, srtCol=20, lhei=c(1,8), #cexRow=1.2, # Increase row label font size cexCol=1.7, # Increase column label font size margin=c(7,1) ) dev.off() #### cluster members ##### write.csv(names(subset(mycl, mycl == '1')),file='YELLOW.txt') write.csv(names(subset(mycl, mycl == '2')),file='BLUE.txt') write.csv(names(subset(mycl, mycl == '3')),file='ORANGE.txt') #~/Tools/csv2xls-0.4/csv_to_xls.py gene_expression_keeping_replicates.txt YELLOW.txt ORANGE.txt BLUE.txt -d',' -o piRNA_heatmap_keeping_replicates.xls mv piRNA_heatmap_keeping_replicates.png piRNA_heatmap.png mv piRNA_heatmap_keeping_replicates.xls piRNA_heatmap.xls mv pca.png piRNA_pca.png mv EV_vs_parental.xls piRNA_EV_vs_parental.xls # --> SENDING piRNA_pca.png, piRNA_EV_vs_parental.xls, piRNA_heatmap.png and piRNA_heatmap.xls # ---- NOT WORKING WELL ---- # merging replicates datamat <- cbind(datamat, "WaGa wildtype" = rowMeans(datamat[, 2:3])) datamat <- cbind(datamat, "WaGa sT DMSO" = rowMeans(datamat[, 4:5])) datamat <- cbind(datamat, "WaGa sT Dox" = rowMeans(datamat[, 6:7])) datamat <- cbind(datamat, "WaGa scr DMSO" = rowMeans(datamat[, 8:9])) datamat <- cbind(datamat, "WaGa scr Dox" = rowMeans(datamat[, 10:11])) datamat <- datamat[,c(-2:-11)] write.csv(datamat, file ="gene_expression_merging_replicates.txt") # Ensure 'mycl' is calculated properly. mycl <- cutree(hr, h=max(hr$height)/2.2) # TODO: Rearrange the colors of the plot *_merging_replicates.png to match those in *_keeping_replicates.png! # mycol = c("YELLOW", "BLUE", "ORANGE", "CYAN", "GREEN", "MAGENTA", "GREY", "LIGHTCYAN", "RED", "PINK", "DARKORANGE", "MAROON", "LIGHTGREEN", "DARKBLUE", "DARKRED", "LIGHTBLUE", "DARKCYAN", "DARKGREEN", "DARKMAGENTA"); # Now map your clusters to colors, making sure that there's one color for each row: actualColors <- mycol[mycl] # Assign colors based on cluster assignment # Then use these 'actualColors' in your heatmap: png("piRNA_heatmap_merging_replicates.png", width=800, height=1000) heatmap.2(as.matrix(datamat), Rowv=as.dendrogram(hr), Colv=NA, dendrogram='row', labRow="", scale='row', trace='none', col=bluered(75), RowSideColors=actualColors, # Update this part srtCol=20, lhei=c(1,8), cexCol=1.7, # Increase column label font size margin=c(7,1) ) dev.off() #### cluster members ##### write.csv(names(subset(mycl, mycl == '1')),file='YELLOW.txt') write.csv(names(subset(mycl, mycl == '2')),file='BLUE.txt') write.csv(names(subset(mycl, mycl == '3')),file='ORANGE.txt') write.csv(names(subset(mycl, mycl == '4')),file='CYAN.txt') write.csv(names(subset(mycl, mycl == '5')),file='GREEN.txt') write.csv(names(subset(mycl, mycl == '6')),file='MAGENTA.txt') write.csv(names(subset(mycl, mycl == '7')),file='GREY.txt') write.csv(names(subset(mycl, mycl == '8')),file='LIGHTCYAN.txt') #write.csv(names(subset(mycl, mycl == '9')),file='RED.txt') #~/Tools/csv2xls-0.4/csv_to_xls.py gene_expression_merging_replicates.txt YELLOW.txt BLUE.txt ORANGE.txt CYAN.txt MAGENTA.txt GREEN.txt LIGHTCYAN.txt GREY.txt -d',' -o piRNA_heatmap_merging_replicates.xls
点赞本文的读者
还没有人对此文章表态
本文有评论
没有评论
看文章,发评论,不要沉默
最受欢迎文章
- Motif Discovery in Biological Sequences: A Comparison of MEME and HOMER
- Calling peaks using findPeaks of HOMER
- Kraken2 Installation and Usage Guide
- Why Do Significant Gene Lists Change After Adding Additional Conditions in Differential Gene Expression Analysis?
- Should the inputs for GSVA be normalized or raw?
- PiCRUST2 Pipeline for Functional Prediction and Pathway Analysis in Metagenomics
- Updating Human Gene Identifiers using Ensembl BioMart: A Step-by-Step Guide
- pheatmap vs heatmap.2
- Setup conda environments
- Guide to Submitting Data to GEO (Gene Expression Omnibus)
最新文章
- Risks of Rebooting into Rescue Mode
- 足突(Podosome)、胞外囊泡(Extracellular Vesicle)与基质金属蛋白酶(MMPs)综合解析
- NCBI BioSample Submission Strategy for PJI and Nasal Microbiota Study
- Human RNA-seq processing for Data_Ben_RNAseq_2025
最多评论文章
- Updating Human Gene Identifiers using Ensembl BioMart: A Step-by-Step Guide
- The top 10 genes
- Retrieving KEGG Genes Using Bioservices in Python
推荐相似文章
Processing Data_Michelle_RNAseq_2025
Setup the environment for lumicks-pylake and C_Trap-Multimer-photontrack.ipynb
🧬 Cadmium Resistance Gene Analysis in Staphylococcus epidermidis HD46
Workflow for RNA-Binding Protein Enrichment and RNA Type Distribution Analysis (Ute’s Project)