Ute RNA-Seq Data Methods
gene_x 0 like s 668 view s
Tags: pipeline
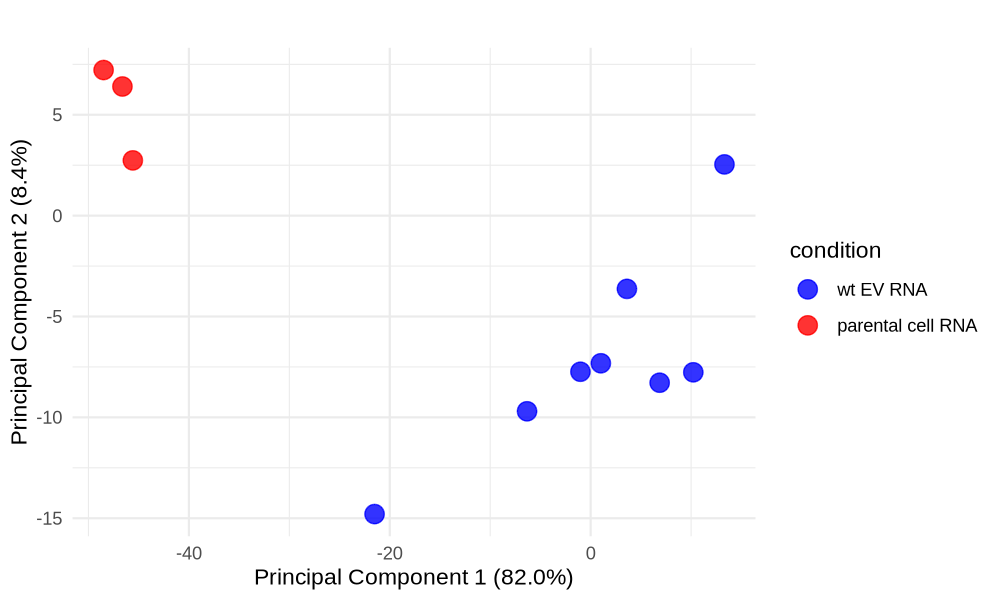
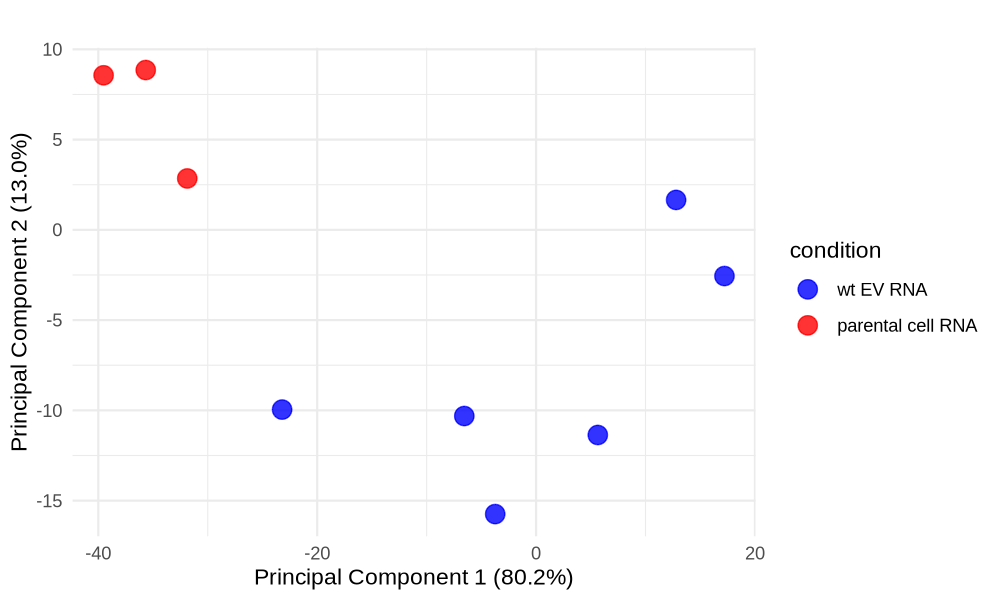
PCA plot untreated/wt vs parental cells; 1x für WaGa cell line und 1x für MKL-1 cells
Heatmap untreated/wt vs parental; 1x für WaGa cell line und 1x für MKL-1 cells
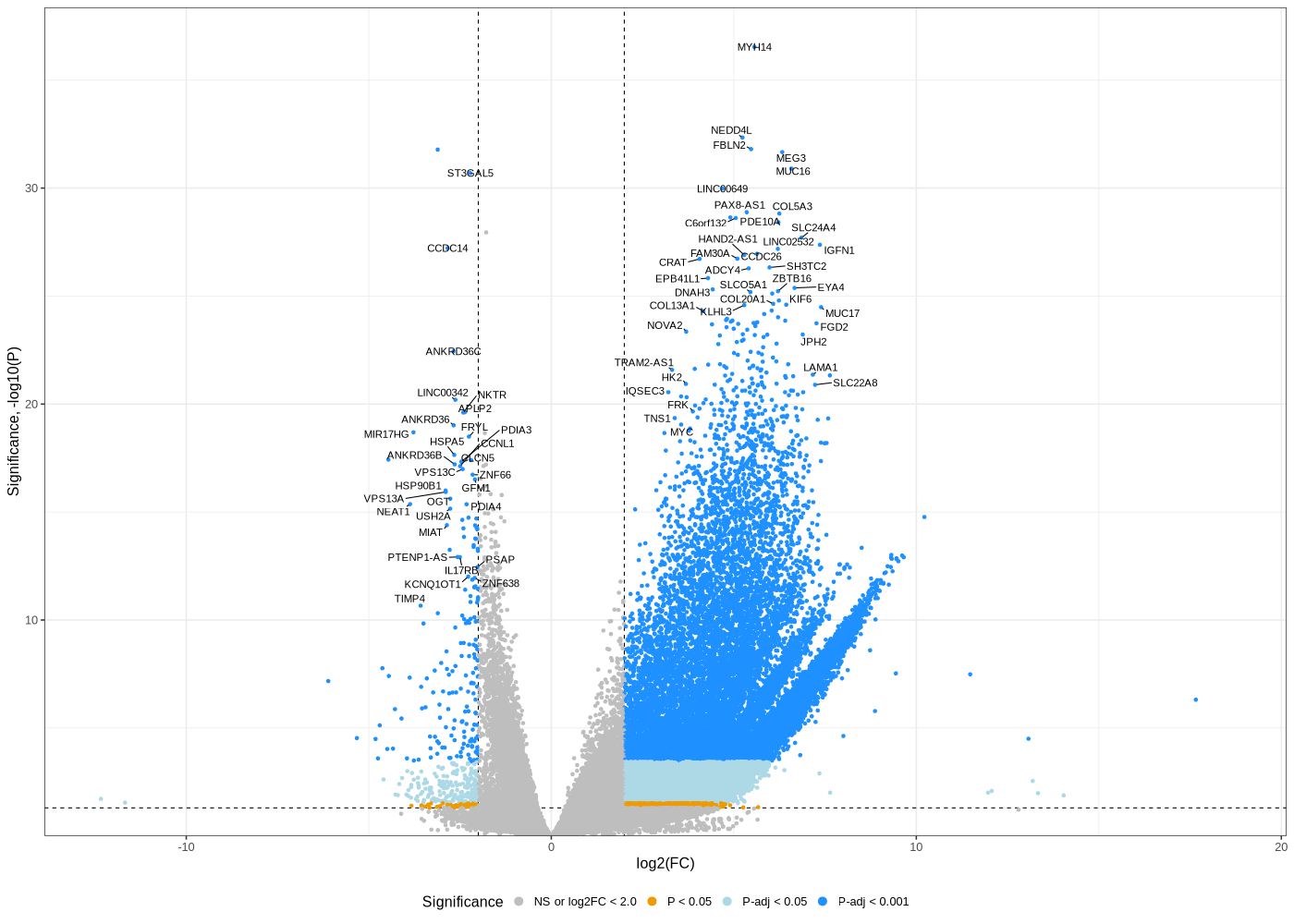
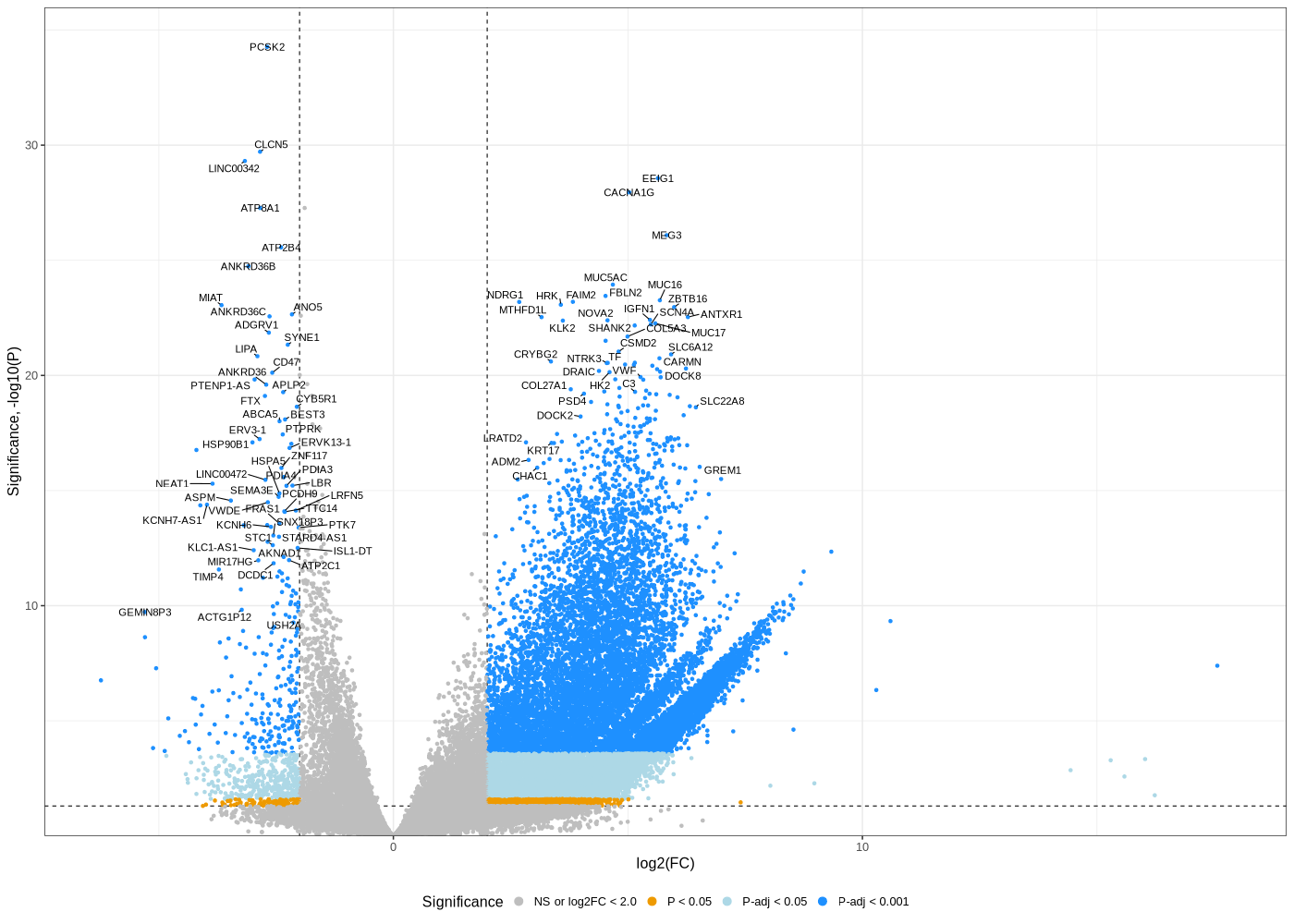
Volcano plot untreated/wt vs parental; 1x für WaGa cell line und 1x für MKL-1 cells
Distribution of different RNA Species untreated/wt and parental; 1x für WaGa cell line und 1x für MKL-1 cells
RNA fragmentation patterns: is EV-RNA full length or fragmented?
RNA binding protein motifs: do we find specific motifs in EV-RNA?
miRNA target analysis
-
Input files (use R 4.3.3)
~/DATA/Data_Ute/Data_RNA-Seq_MKL-1+WaGa/merged_gene_counts_40samples.txt ~/DATA/Data_Ute/Data_RNA-Seq_MKL-1+WaGa/merged_gene_counts_virus_rounded.txt -
Common processing for the data MKL+1 + WaGa
#[1] "/home/jhuang/mambaforge/envs/r_env/lib/R/library" setwd("/home/jhuang/DATA/Data_Ute/Data_RNA-Seq_MKL-1_WaGa/results_2025_1/") library("AnnotationDbi") library("clusterProfiler") library("ReactomePA") #library("org.Mm.eg.db") library(DESeq2) library(gplots) d.raw_human<- read.delim2("../merged_gene_counts_40samples.txt",sep="\t", header=TRUE, row.names=1) colnames(d.raw_human)<- c("gene_name","X042_MKL.1_wt_EV","MKL.1_RNA_147","X042_MKL.1_sT_DMSO","MKL.1_RNA","X0505_MKL.1_scr_DMSO_EV","X0505_MKL.1_sT_DMSO_EV","MKL.1_EV.RNA_87","MKL.1_EV.RNA_27","X042_MKL.1_scr_Dox_EV","X042_MKL.1_scr_DMSO_EV","MKL.1_EV.RNA_118","X0505_MKL.1_scr_Dox_EV","MKL.1_EV.RNA","X042_MKL.1_sT_Dox","X0505_MKL.1_sT_Dox_EV","MKL.1_RNA_118","MKL.1_EV.RNA_2", "Geneid.1","gene_name.1","X1605_WaGa_sT_DMSO_EV","WaGa_EV.RNA_118","WaGa_EV.RNA","X2706_WaGa_scr_Dox_EV","X2706_WaGa_sT_DMSO_EV","X1107_WaGa_wt_EV","X1107_WaGa_sT_Dox_EV","WaGa_RNA","X1605_WaGa_scr_DMSO_EV","X2706_WaGa_scr_DMSO_EV","WaGa_EV.RNA_226","X2706_WaGa_sT_Dox_EV","X1605_WaGa_scr_Dox_EV","X1605_WaGa_wt_EV","X1605_WaGa_sT_Dox_EV","X1107_WaGa_scr_DMSO_EV","WaGa_EV.RNA_2","WaGa_EV.RNA_147","WaGa_RNA_118","X1107_WaGa_scr_Dox_EV","X1107_WaGa_sT_DMSO_EV","WaGa_RNA_147","X2706_WaGa_wt_EV") col_order <- c("gene_name", "MKL.1_RNA","MKL.1_RNA_118","MKL.1_RNA_147","MKL.1_EV.RNA","MKL.1_EV.RNA_2","MKL.1_EV.RNA_118","MKL.1_EV.RNA_87","MKL.1_EV.RNA_27","X042_MKL.1_wt_EV","X042_MKL.1_sT_DMSO","X0505_MKL.1_sT_DMSO_EV","X042_MKL.1_scr_DMSO_EV","X0505_MKL.1_scr_DMSO_EV","X042_MKL.1_sT_Dox","X0505_MKL.1_sT_Dox_EV","X042_MKL.1_scr_Dox_EV","X0505_MKL.1_scr_Dox_EV", "Geneid.1","gene_name.1", "WaGa_RNA","WaGa_RNA_118","WaGa_RNA_147", "WaGa_EV.RNA","WaGa_EV.RNA_2","WaGa_EV.RNA_118","WaGa_EV.RNA_147","WaGa_EV.RNA_226","X1107_WaGa_wt_EV","X1605_WaGa_wt_EV","X2706_WaGa_wt_EV", "X1107_WaGa_sT_DMSO_EV","X1605_WaGa_sT_DMSO_EV","X2706_WaGa_sT_DMSO_EV", "X1107_WaGa_scr_DMSO_EV","X1605_WaGa_scr_DMSO_EV","X2706_WaGa_scr_DMSO_EV", "X1107_WaGa_sT_Dox_EV","X1605_WaGa_sT_Dox_EV","X2706_WaGa_sT_Dox_EV", "X1107_WaGa_scr_Dox_EV","X1605_WaGa_scr_Dox_EV","X2706_WaGa_scr_Dox_EV") reordered.raw_human <- d.raw_human[,col_order] d.raw_virus <- read.delim2("../merged_gene_counts_virus_rounded.txt",sep="\t", header=TRUE, row.names=1) reordered.raw_virus <- d.raw_virus[,col_order] identical(colnames(reordered.raw_human), colnames(reordered.raw_virus)) reordered.raw <- rbind(reordered.raw_human, reordered.raw_virus) #rename colnames(reordered.raw) <- c("gene_name", "MKL-1 parental cell RNA","MKL-1 parental cell RNA 118","MKL-1 parental cell RNA 147", "MKL-1 wt EV RNA","MKL-1 wt EV RNA 2","MKL-1 wt EV RNA 118","MKL-1 wt EV RNA 87","MKL-1 wt EV RNA 27","MKL-1 wt EV RNA 042", "MKL-1 sT DMSO EV RNA 042","MKL-1 sT DMSO EV RNA 0505", "MKL-1 scr DMSO EV RNA 042","MKL-1 scr DMSO EV RNA 0505", "MKL-1 sT Dox EV RNA 042","MKL-1 sT Dox EV RNA 0505", "MKL-1 scr Dox EV RNA 042","MKL-1 scr Dox EV RNA 0505", "Geneid.1","gene_name.1", "WaGa parental cell RNA","WaGa parental cell RNA 118","WaGa parental cell RNA 147", "WaGa wt EV RNA","WaGa wt EV RNA 2","WaGa wt EV RNA 118","WaGa wt EV RNA 147","WaGa wt EV RNA 226","WaGa wt EV RNA 1107","WaGa wt EV RNA 1605","WaGa wt EV RNA 2706", "WaGa sT DMSO EV RNA 1107","WaGa sT DMSO EV RNA 1605","WaGa sT DMSO EV RNA 2706", "WaGa scr DMSO EV RNA 1107","WaGa scr DMSO EV RNA 1605","WaGa scr DMSO EV RNA 2706", "WaGa sT Dox EV RNA 1107","WaGa sT Dox EV RNA 1605","WaGa sT Dox EV RNA 2706", "WaGa scr Dox EV RNA 1107","WaGa scr Dox EV RNA 1605","WaGa scr Dox EV RNA 2706") reordered.raw$gene_name <- NULL reordered.raw$Geneid.1 <- NULL reordered.raw$gene_name.1 <- NULL write.csv(reordered.raw, file="counts.txt") #IMPORTANT that we should filter the data with the counts in the STEP! d <- reordered.raw[rowSums(reordered.raw>3)>2,] condition_for_pca = as.factor(c("RNA","RNA","RNA","EV","EV","EV","EV","EV","EV","sT.DMSO","sT.DMSO","scr.DMSO","scr.DMSO","sT.Dox","sT.Dox","scr.Dox","scr.Dox", "RNA","RNA","RNA","EV","EV","EV","EV","EV","EV","EV","EV","sT.DMSO","sT.DMSO","sT.DMSO","scr.DMSO","scr.DMSO","scr.DMSO","sT.Dox","sT.Dox","sT.Dox","scr.Dox","scr.Dox","scr.Dox")) condition = as.factor(c("MKL1.RNA","MKL1.RNA","MKL1.RNA","MKL1.EV","MKL1.EV","MKL1.EV","MKL1.EV","MKL1.EV","MKL1.EV","MKL1.sT.DMSO","MKL1.sT.DMSO","MKL1.scr.DMSO","MKL1.scr.DMSO","MKL1.sT.Dox","MKL1.sT.Dox","MKL1.scr.Dox","MKL1.scr.Dox", "WaGa.RNA","WaGa.RNA","WaGa.RNA","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.sT.DMSO","WaGa.sT.DMSO","WaGa.sT.DMSO","WaGa.scr.DMSO","WaGa.scr.DMSO","WaGa.scr.DMSO","WaGa.sT.Dox","WaGa.sT.Dox","WaGa.sT.Dox","WaGa.scr.Dox","WaGa.scr.Dox","WaGa.scr.Dox")) donor = as.factor(c("1","118","147", "1","2","118","87","27","042", "042","0505","042","0505","042","0505","042","0505", "1","118","147", "1","2","118","147","226","1107","1605","2706", "1107","1605","2706","1107","1605","2706","1107","1605","2706","1107","1605","2706")) batch = as.factor(c("2021.08","2021.09","2021.09","2021.08","2021.08","2021.09","2021.09","2021.09","2022.08","2022.08","2022.08","2022.08","2022.08","2022.08","2022.08","2022.08","2022.08", "2021.08","2021.09","2021.09","2021.08","2021.08","2021.09","2021.09","2021.09","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11")) cell.line = as.factor(c("MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1", "WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa")) ids = as.factor(c("MKL1.RNA","MKL1.RNA.118","MKL1.RNA.147", "MKL1.EV","MKL1.EV.2","MKL1.EV.118","MKL1.EV.87","MKL1.EV.27","MKL1.EV.042", "MKL1.EV.sT.DMSO.042","MKL1.EV.sT.DMSO.0505", "MKL1.EV.scr.DMSO.042","MKL1.EV.scr.DMSO.0505", "MKL1.EV.sT.Dox.042","MKL1.EV.sT.Dox.0505", "MKL1.EV.scr.Dox.042","MKL1.EV.scr.Dox.0505", "WaGa.RNA","WaGa.RNA.118","WaGa.RNA.147", "WaGa.EV","WaGa.EV.2","WaGa.EV.118","WaGa.EV.147","WaGa.EV.226","WaGa.EV.1107","WaGa.EV.1605","WaGa.EV.2706", "WaGa.EV.sT.DMSO.1107","WaGa.EV.sT.DMSO.1605","WaGa.EV.sT.DMSO.2706", "WaGa.EV.scr.DMSO.1107","WaGa.EV.scr.DMSO.1605","WaGa.EV.scr.DMSO.2706", "WaGa.EV.sT.Dox.1107","WaGa.EV.sT.Dox.1605","WaGa.EV.sT.Dox.2706", "WaGa.EV.scr.Dox.1107","WaGa.EV.scr.Dox.1605","WaGa.EV.scr.Dox.2706")) cData = data.frame(row.names=colnames(d), condition=condition, donor=donor, batch=batch, cell.line=cell.line, ids=ids) dds<-DESeqDataSetFromMatrix(countData=d, colData=cData, design=~batch+condition) #rld <- rlogTransformation(dds) rld <- vst(dds) -
Preparing the data for PCA_MKL1 and PCA_WaGa drawing
#d_WaGa <- d[, !grepl("parental|MKL-1", names(d))] d_WaGa <- d[, !grepl("MKL-1", names(d))] condition = as.factor(c("WaGa.RNA","WaGa.RNA","WaGa.RNA","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.EV","WaGa.sT.DMSO","WaGa.sT.DMSO","WaGa.sT.DMSO","WaGa.scr.DMSO","WaGa.scr.DMSO","WaGa.scr.DMSO","WaGa.sT.Dox","WaGa.sT.Dox","WaGa.sT.Dox","WaGa.scr.Dox","WaGa.scr.Dox","WaGa.scr.Dox")) donor = as.factor(c("1","118","147","1","2","118","147","226","1107","1605","2706", "1107","1605","2706","1107","1605","2706","1107","1605","2706","1107","1605","2706")) batch = as.factor(c("2021.08","2021.09","2021.09","2021.08","2021.08","2021.09","2021.09","2021.09","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11","2022.11")) cell.line = as.factor(c("WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa","WaGa")) ids = as.factor(c("WaGa.RNA","WaGa.RNA.118","WaGa.RNA.147","WaGa.EV","WaGa.EV.2","WaGa.EV.118","WaGa.EV.147","WaGa.EV.226","WaGa.EV.1107","WaGa.EV.1605","WaGa.EV.2706", "WaGa.EV.sT.DMSO.1107","WaGa.EV.sT.DMSO.1605","WaGa.EV.sT.DMSO.2706", "WaGa.EV.scr.DMSO.1107","WaGa.EV.scr.DMSO.1605","WaGa.EV.scr.DMSO.2706", "WaGa.EV.sT.Dox.1107","WaGa.EV.sT.Dox.1605","WaGa.EV.sT.Dox.2706", "WaGa.EV.scr.Dox.1107","WaGa.EV.scr.Dox.1605","WaGa.EV.scr.Dox.2706")) cData = data.frame(row.names=colnames(d_WaGa), condition=condition, donor=donor, batch=batch, cell.line=cell.line, ids=ids) dds_WaGa<-DESeqDataSetFromMatrix(countData=d_WaGa, colData=cData, design=~batch+condition) rld_WaGa <- vst(dds_WaGa) #d_MKL1 <- d[, !grepl("parental|WaGa", names(d))] d_MKL1 <- d[, !grepl("WaGa", names(d))] condition = as.factor(c("MKL1.RNA","MKL1.RNA","MKL1.RNA","MKL1.EV","MKL1.EV","MKL1.EV","MKL1.EV","MKL1.EV","MKL1.EV","MKL1.sT.DMSO","MKL1.sT.DMSO","MKL1.scr.DMSO","MKL1.scr.DMSO","MKL1.sT.Dox","MKL1.sT.Dox","MKL1.scr.Dox","MKL1.scr.Dox")) donor = as.factor(c("1","118","147","1","2","118","87","27","042", "042","0505","042","0505","042","0505","042","0505")) batch = as.factor(c("2021.08","2021.09","2021.09","2021.08","2021.08","2021.09","2021.09","2021.09","2022.08","2022.08","2022.08","2022.08","2022.08","2022.08","2022.08","2022.08","2022.08")) cell.line = as.factor(c("MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1","MKL-1")) ids = as.factor(c("MKL1.RNA","MKL1.RNA.118","MKL1.RNA.147", "MKL1.EV","MKL1.EV.2","MKL1.EV.118","MKL1.EV.87","MKL1.EV.27","MKL1.EV.042", "MKL1.EV.sT.DMSO.042","MKL1.EV.sT.DMSO.0505", "MKL1.EV.scr.DMSO.042","MKL1.EV.scr.DMSO.0505", "MKL1.EV.sT.Dox.042","MKL1.EV.sT.Dox.0505", "MKL1.EV.scr.Dox.042","MKL1.EV.scr.Dox.0505")) cData = data.frame(row.names=colnames(d_MKL1), condition=condition, donor=donor, batch=batch, cell.line=cell.line, ids=ids) dds_MKL1<-DESeqDataSetFromMatrix(countData=d_MKL1, colData=cData, design=~batch+condition) rld_MKL1 <- vst(dds_MKL1) # -- before pca -- png("pca.png", 1200, 800) plotPCA(rld, intgroup=c("condition")) #plotPCA(rld, intgroup = c("condition", "batch")) #plotPCA(rld, intgroup = c("condition", "ids")) #plotPCA(rld, "batch") dev.off() #72% (PC1), 11% (PC2) 8% (PC3) png("pca_WaGa.png", 1200, 800) plotPCA(rld_WaGa, intgroup = c("condition")) dev.off() #80%, 9% png("pca_MKL1.png", 1200, 800) plotPCA(rld_MKL1, intgroup = c("condition")) dev.off() #77%, 15% -
PCA plot untreated/wt vs parental cells; 1x für WaGa cell line und 1x für MKL-1 cells
#install.packages("BiocManager") #BiocManager::install("genefilter") #install.packages("writexl") #install.packages("plotly") #install.packages("dplyr") #install.packages("ggrepel") # Load required libraries library(ggrepel) library(dplyr) library(ggplot2) library(genefilter) library(writexl) # ---- drawing PCA WaGa ---- # Perform PCA on WaGa dataset pca_data <- plotPCA(rld_WaGa, intgroup = c("condition", "batch", "cell.line"), returnData = TRUE) write.csv(pca_data, file = "PCA_data_WaGa.csv") # Compute top variable genes and perform PCA top_variable_genes <- 500 gene_variance <- rowVars(assay(rld_WaGa)) selected_genes <- order(gene_variance, decreasing = TRUE)[seq_len(min(top_variable_genes, length(gene_variance)))] # Extract data and run PCA expression_matrix <- t(assay(rld_WaGa)[selected_genes, ]) pca_results <- prcomp(expression_matrix, center = TRUE, scale. = TRUE) summary(pca_results) # Display variance explained by PCs # Extract variance percentages variance_explained <- summary(pca_results)$importance[2, ] pc1_variance <- sprintf("%.1f", variance_explained[1] * 100) pc2_variance <- sprintf("%.1f", variance_explained[2] * 100) # Create a PCA dataframe pca_df <- as.data.frame(pca_results$x) rownames(pca_df) <- rownames(pca_data) # Merge PCA results with original metadata pca_data <- pca_data %>% select(-PC1, -PC2) # Remove old PCA values merged_pca_data <- merge(pca_data, pca_df, by = "row.names") rownames(merged_pca_data) <- merged_pca_data$Row.names merged_pca_data$Row.names <- NULL # Save merged PCA data write.csv(merged_pca_data, file = "Merged_PCA_WaGa.csv") write_xlsx(merged_pca_data, path = "Merged_PCA_WaGa.xlsx") # Define condition labels condition_labels <- c( "WaGa.EV" = "wt EV RNA", "WaGa.scr.DMSO" = "scr DMSO EV RNA", "WaGa.scr.Dox" = "scr Dox EV RNA", "WaGa.sT.DMSO" = "sT DMSO EV RNA", "WaGa.sT.Dox" = "sT Dox EV RNA", "WaGa.RNA" = "parental cell RNA" ) # Apply condition labels and filter relevant conditions filtered_pca_data <- merged_pca_data %>% mutate(condition = recode(condition, !!!condition_labels)) %>% filter(condition %in% c("wt EV RNA", "parental cell RNA")) # Prepare PCA data for plotting plot_pca_df <- as.data.frame(filtered_pca_data[, c("PC1", "PC2")]) plot_pca_df$condition <- filtered_pca_data$condition plot_pca_df$sample_name <- rownames(filtered_pca_data) # Save PCA plot as PNG png("PCA_WaGa.png", width = 1000, height = 600, res = 150) ggplot(plot_pca_df, aes(x = PC1, y = PC2, color = condition)) + geom_point(size = 4, alpha = 0.8) + # Plot points labs( title = "", x = paste0("Principal Component 1 (", pc1_variance, "%)"), y = paste0("Principal Component 2 (", pc2_variance, "%)") ) + theme_minimal() + scale_color_manual(values = c("blue", "red")) # Customize colors dev.off() # Close the PNG device and save the file # ---- drawing PCA MKL-1 ---- # Compute PCA on MKL1 dataset pca_data <- plotPCA(rld_MKL1, intgroup = c("condition", "batch", "cell.line"), returnData = TRUE) write.csv(pca_data, file = "PCA_data_MKL1.csv") # Compute top variable genes and perform PCA top_variable_genes <- 500 gene_variance <- rowVars(assay(rld_MKL1)) selected_genes <- order(gene_variance, decreasing = TRUE)[seq_len(min(top_variable_genes, length(gene_variance)))] # Extract and transpose data for PCA expression_matrix <- t(assay(rld_MKL1)[selected_genes, ]) pca_results <- prcomp(expression_matrix, center = TRUE, scale. = TRUE) # Extract variance explained percentages variance_explained <- summary(pca_results)$importance[2, ] pc1_variance <- sprintf("%.1f", variance_explained[1] * 100) pc2_variance <- sprintf("%.1f", variance_explained[2] * 100) # Create a PCA dataframe pca_df <- as.data.frame(pca_results$x) rownames(pca_df) <- rownames(pca_data) # Remove previous PC1 & PC2 columns and merge new PCA results pca_data <- pca_data %>% select(-PC1, -PC2) merged_pca_data <- merge(pca_data, pca_df, by = "row.names") rownames(merged_pca_data) <- merged_pca_data$Row.names merged_pca_data$Row.names <- NULL # Save merged PCA data write.csv(merged_pca_data, file = "Merged_PCA_MKL1.csv") write_xlsx(merged_pca_data, path = "Merged_PCA_MKL1.xlsx") # Define condition labels for clarity condition_labels <- c( "MKL1.EV" = "wt EV RNA", "MKL1.scr.DMSO" = "scr DMSO EV RNA", "MKL1.scr.Dox" = "scr Dox EV RNA", "MKL1.sT.DMSO" = "sT DMSO EV RNA", "MKL1.sT.Dox" = "sT Dox EV RNA", "MKL1.RNA" = "parental cell RNA" ) # Apply condition labels and filter for relevant conditions filtered_pca_data <- merged_pca_data %>% mutate(condition = recode(condition, !!!condition_labels)) %>% filter(condition %in% c("wt EV RNA", "parental cell RNA")) # Prepare PCA data for plotting plot_pca_df <- as.data.frame(filtered_pca_data[, c("PC1", "PC2")]) plot_pca_df$condition <- filtered_pca_data$condition plot_pca_df$sample_name <- rownames(filtered_pca_data) # Save PCA plot as PNG png("PCA_MKL1.png", width = 1000, height = 600, res = 150) ggplot(plot_pca_df, aes(x = PC1, y = PC2, color = condition)) + geom_point(size = 4, alpha = 0.8) + # Plot points labs( title = "", x = paste0("Principal Component 1 (", pc1_variance, "%)"), y = paste0("Principal Component 2 (", pc2_variance, "%)") ) + theme_minimal() + scale_color_manual(values = c("blue", "red")) # Customize colors dev.off() # Close the PNG device and save the file -
Convert bam to bigwig using deepTools by feeding inverse of DESeq’s size Factor
sizeFactors(dds) #NULL dds <- estimateSizeFactors(dds) > sizeFactors(dds) #WaGa parental cell RNA* # 1.1242096 2.1097851 #WaGa parental cell RNA 118* WaGa parental cell RNA 147* # 1.6925780 1.6712182 raw_counts <- counts(dds) normalized_counts <- counts(dds, normalized=TRUE) write.table(raw_counts, file="raw_counts.txt", sep="\t", quote=F, col.names=NA) write.table(normalized_counts, file="normalized_counts.txt", sep="\t", quote=F, col.names=NA) #1/2,1097851=0,473981924 (!=0.4958732) bamCoverage --bam ../markDuplicates/WaGa_RNAAligned.sortedByCoord.out.markDups.bam -o WaGa_RNA.bw --binSize 10 --scaleFactor 0.473981924 --effectiveGenomeSize 2864785220 #1/1,6925780=0,590814722 (!=0.6013898) bamCoverage --bam ../markDuplicates/WaGa_RNA_118Aligned.sortedByCoord.out.markDups.bam -o WaGa_RNA_118.bw --binSize 10 --scaleFactor 0.590814722 --effectiveGenomeSize 2864785220 #1/1,6712182=0,598365911 (!=0.6154516) bamCoverage --bam ../markDuplicates/WaGa_RNA_147Aligned.sortedByCoord.out.markDups.bam -o WaGa_RNA_147.bw --binSize 10 --scaleFactor 0.598365911 --effectiveGenomeSize 2864785220 -
DEG analysis
##https://bioconductor.statistik.tu-dortmund.de/packages/3.7/data/annotation/ #BiocManager::install("EnsDb.Mmusculus.v79") #library(EnsDb.Mmusculus.v79) #edb <- EnsDb.Mmusculus.v79 #https://bioconductor.org/packages/release/bioc/vignettes/biomaRt/inst/doc/accessing_ensembl.html#selecting-an-ensembl-biomart-database-and-dataset #https://bioconductor.org/packages/release/bioc/vignettes/biomaRt/inst/doc/accessing_ensembl.html#selecting-an-ensembl-biomart-database-and-dataset library(biomaRt) #listMarts() == listEnsembl() == Ensembl Genes 113 listEnsembl() listMarts() listEnsemblArchives() #mkdir degenes setwd("degenes") dds$condition <- relevel(dds$condition, "WaGa.RNA") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("WaGa.EV_vs_WaGa.RNA") dds$condition <- relevel(dds$condition, "MKL1.RNA") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("MKL1.EV_vs_MKL1.RNA") #ensembl <- useEnsembl(biomart = "ensembl", dataset = "hsapiens_gene_ensembl", version="112") #ensembl <- useMart("ensembl", dataset="hsapiens_gene_ensembl", host="https://may2023.archive.ensembl.org") ensembl <- useMart("ensembl", dataset="hsapiens_gene_ensembl", host="https://www.ensembl.org") listDatasets(ensembl) attributes = listAttributes(ensembl) attributes[1:25,] for (i in clist) { #i<-clist[1] contrast = paste("condition", i, sep="_") res = results(dds, name=contrast) res <- res[!is.na(res$log2FoldChange),] geness <- getBM(attributes = c('ensembl_gene_id', 'external_gene_name', 'gene_biotype', 'entrezgene_id', 'chromosome_name', 'start_position', 'end_position', 'strand', 'description'), filters = 'ensembl_gene_id', values = rownames(res), mart = ensembl) geness_uniq <- distinct(geness, ensembl_gene_id, .keep_all= TRUE) #merge by column by common colunmn name, in the case "GENEID" res$ENSEMBL = rownames(res) identical(rownames(res), rownames(geness_uniq)) res_df <- as.data.frame(res) geness_res <- merge(geness_uniq, res_df, by.x="ensembl_gene_id", by.y="ENSEMBL") dim(geness_res) rownames(geness_res) <- geness_res$ensembl_gene_id geness_res$ensembl_gene_id <- NULL write.csv(as.data.frame(geness_res[order(geness_res$pvalue),]), file = paste(i, "all.txt", sep="-")) up <- subset(geness_res, padj<=0.05 & log2FoldChange>=2) down <- subset(geness_res, padj<=0.05 & log2FoldChange<=-2) write.csv(as.data.frame(up[order(up$log2FoldChange,decreasing=TRUE),]), file = paste(i, "up.txt", sep="-")) write.csv(as.data.frame(down[order(abs(down$log2FoldChange),decreasing=TRUE),]), file = paste(i, "down.txt", sep="-")) } -
Volcano plot untreated/wt vs parental; 1x für WaGa cell line und 1x für MKL-1 cells
#A canonical visualization for interpreting differential gene expression results is the volcano plot. library(ggrepel) # -- WaGa.EV_vs_WaGa.RNA -- geness_res <- read.csv(file = paste("WaGa.EV_vs_WaGa.RNA", "all.txt", sep="-"), row.names=1) geness_res$Color <- "NS or log2FC < 2.0" geness_res$Color[geness_res$pvalue < 0.05] <- "P < 0.05" geness_res$Color[geness_res$padj < 0.05] <- "P-adj < 0.05" geness_res$Color[geness_res$padj < 0.001] <- "P-adj < 0.001" geness_res$Color[abs(geness_res$log2FoldChange) < 2.0] <- "NS or log2FC < 2.0" geness_res$Color <- factor(geness_res$Color, levels = c("NS or log2FC < 2.0", "P < 0.05", "P-adj < 0.05", "P-adj < 0.001")) geness_res$invert_P <- (-log10(geness_res$pvalue)) * sign(geness_res$log2FoldChange) top_g <- c() top_g <- c(top_g, geness_res[, 'external_gene_name'][order(geness_res[, 'invert_P'], decreasing = TRUE)[1:200]], geness_res[, 'external_gene_name'][order(geness_res[, 'invert_P'], decreasing = FALSE)[1:200]]) top_g <- unique(top_g) geness_res <- geness_res[, -1*ncol(geness_res)] #remove invert_P from matrix png("WaGa_wt.EV_vs_parental.png",width=1400, height=1000) ggplot(geness_res, aes(x = log2FoldChange, y = -log10(pvalue), color = Color, label = external_gene_name)) + geom_vline(xintercept = c(2.0, -2.0), lty = "dashed") + geom_hline(yintercept = -log10(0.05), lty = "dashed") + geom_point() + labs(x = "log2(FC)", y = "Significance, -log10(P)", color = "Significance") + scale_color_manual(values = c(`P-adj < 0.001` = "dodgerblue", `P-adj < 0.05` = "lightblue", `P < 0.05` = "orange2", `NS or log2FC < 2.0` = "gray"), guide = guide_legend(override.aes = list(size = 4))) + scale_y_continuous(expand = expansion(mult = c(0,0.05))) + geom_text_repel(data = subset(geness_res, external_gene_name %in% top_g & pvalue < 0.05 & (abs(geness_res$log2FoldChange) >= 2.0)), size = 4, point.padding = 0.15, color = "black", min.segment.length = .1, box.padding = .2, lwd = 2) + theme_bw(base_size = 16) + theme(legend.position = "bottom") dev.off() # -- MKL1.EV_vs_MKL1.RNA -- geness_res <- read.csv(file = paste("MKL1.EV_vs_MKL1.RNA", "all.txt", sep="-"), row.names=1) geness_res$Color <- "NS or log2FC < 2.0" geness_res$Color[geness_res$pvalue < 0.05] <- "P < 0.05" geness_res$Color[geness_res$padj < 0.05] <- "P-adj < 0.05" geness_res$Color[geness_res$padj < 0.001] <- "P-adj < 0.001" geness_res$Color[abs(geness_res$log2FoldChange) < 2.0] <- "NS or log2FC < 2.0" geness_res$Color <- factor(geness_res$Color, levels = c("NS or log2FC < 2.0", "P < 0.05", "P-adj < 0.05", "P-adj < 0.001")) geness_res$invert_P <- (-log10(geness_res$pvalue)) * sign(geness_res$log2FoldChange) top_g <- c() top_g <- c(top_g, geness_res[, 'external_gene_name'][order(geness_res[, 'invert_P'], decreasing = TRUE)[1:200]], geness_res[, 'external_gene_name'][order(geness_res[, 'invert_P'], decreasing = FALSE)[1:200]]) top_g <- unique(top_g) geness_res <- geness_res[, -1*ncol(geness_res)] #remove invert_P from matrix png("MKL-1_wt.EV_vs_parental.png",width=1400, height=1000) ggplot(geness_res, aes(x = log2FoldChange, y = -log10(pvalue), color = Color, label = external_gene_name)) + geom_vline(xintercept = c(2.0, -2.0), lty = "dashed") + geom_hline(yintercept = -log10(0.05), lty = "dashed") + geom_point() + labs(x = "log2(FC)", y = "Significance, -log10(P)", color = "Significance") + scale_color_manual(values = c(`P-adj < 0.001` = "dodgerblue", `P-adj < 0.05` = "lightblue", `P < 0.05` = "orange2", `NS or log2FC < 2.0` = "gray"), guide = guide_legend(override.aes = list(size = 4))) + scale_y_continuous(expand = expansion(mult = c(0,0.05))) + geom_text_repel(data = subset(geness_res, external_gene_name %in% top_g & pvalue < 0.05 & (abs(geness_res$log2FoldChange) >= 2.0)), size = 4, point.padding = 0.15, color = "black", min.segment.length = .1, box.padding = .2, lwd = 2) + theme_bw(base_size = 16) + theme(legend.position = "bottom") dev.off() # -- under console -- mv WaGa.EV_vs_WaGa.RNA-all.txt WaGa_wt.EV_vs_parental-all.txt mv WaGa.EV_vs_WaGa.RNA-up.txt WaGa_wt.EV_vs_parental-up.txt mv WaGa.EV_vs_WaGa.RNA-down.txt WaGa_wt.EV_vs_parental-down.txt mv MKL1.EV_vs_MKL1.RNA-all.txt MKL-1_wt.EV_vs_parental-all.txt mv MKL1.EV_vs_MKL1.RNA-up.txt MKL-1_wt.EV_vs_parental-up.txt mv MKL1.EV_vs_MKL1.RNA-down.txt MKL-1_wt.EV_vs_parental-down.txt for cmp in "WaGa_wt.EV_vs_parental" "MKL-1_wt.EV_vs_parental"; do echo "~/Tools/csv2xls-0.4/csv_to_xls.py ${cmp}-all.txt ${cmp}-up.txt ${cmp}-down.txt -d$',' -o ${cmp}.xls" done ~/Tools/csv2xls-0.4/csv_to_xls.py WaGa_wt.EV_vs_parental-all.txt WaGa_wt.EV_vs_parental-up.txt WaGa_wt.EV_vs_parental-down.txt -d$',' -o WaGa_wt.EV_vs_parental.xls ~/Tools/csv2xls-0.4/csv_to_xls.py MKL-1_wt.EV_vs_parental-all.txt MKL-1_wt.EV_vs_parental-up.txt MKL-1_wt.EV_vs_parental-down.txt -d$',' -o MKL-1_wt.EV_vs_parental.xls -
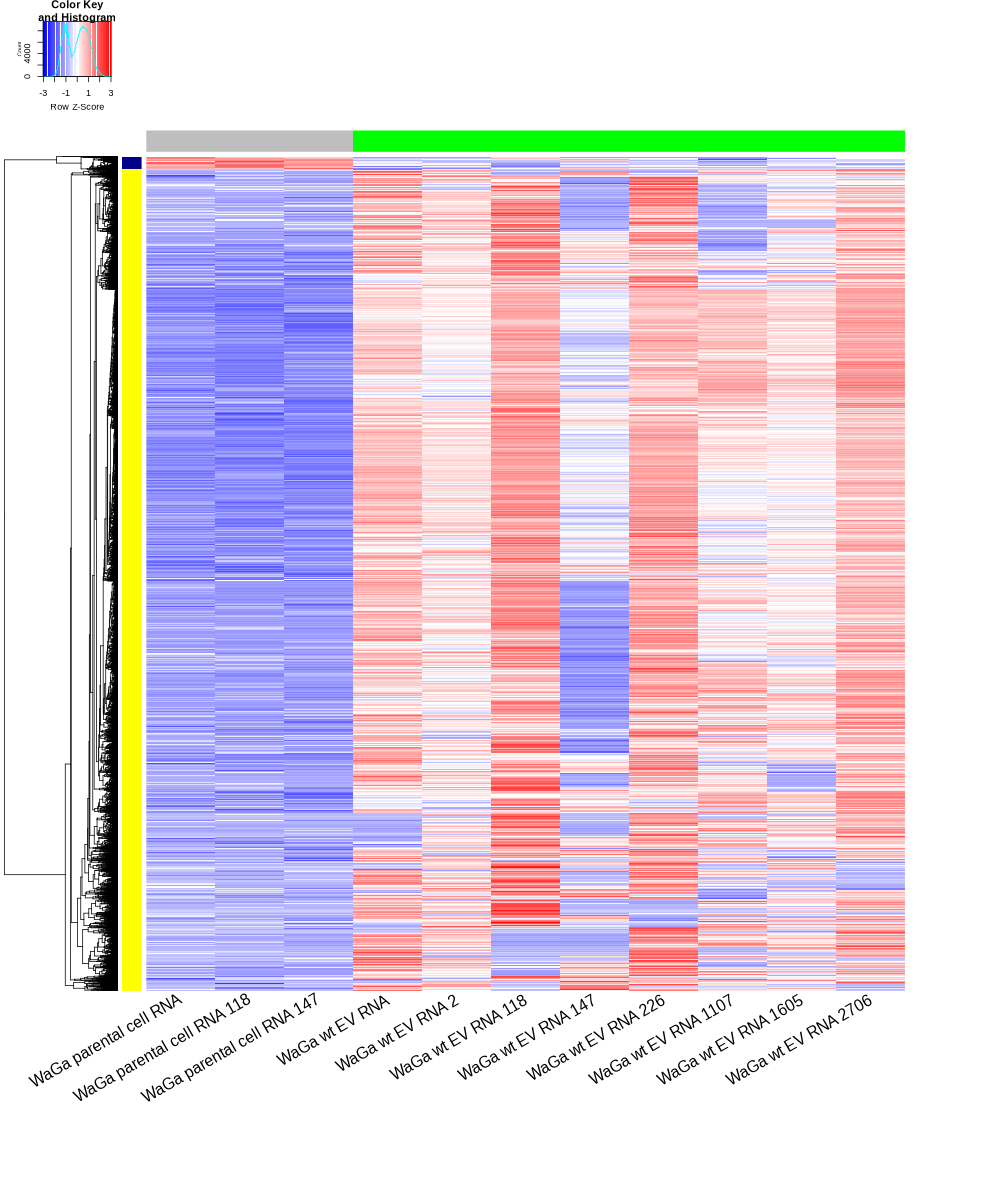
Heatmap untreated/wt vs parental; 1x für WaGa cell line und 1x für MKL-1 cells
install.packages("gplots") library("gplots") # -- WaGa cell line -- cut -d',' -f1-1 ./WaGa_wt.EV_vs_parental-up.txt > WaGa_wt.EV_vs_parental-up.id cut -d',' -f1-1 ./WaGa_wt.EV_vs_parental-down.txt > WaGa_wt.EV_vs_parental-down.id cat WaGa_wt.EV_vs_parental-up.id WaGa_wt.EV_vs_parental-down.id | sort -u > ids #24207 #add Gene_Id in the first line. GOI <- read.csv("ids")$Gene_Id RNASeq.NoCellLine <- assay(rld) #clustering methods: "ward.D", "ward.D2", "single", "complete", "average" (= UPGMA), "mcquitty" (= WPGMA), "median" (= WPGMC) or "centroid" (= UPGMC). pearson or spearman datamat = RNASeq.NoCellLine[GOI, c("WaGa parental cell RNA","WaGa parental cell RNA 118","WaGa parental cell RNA 147", "WaGa wt EV RNA","WaGa wt EV RNA 2","WaGa wt EV RNA 118","WaGa wt EV RNA 147","WaGa wt EV RNA 226", "WaGa wt EV RNA 1107","WaGa wt EV RNA 1605","WaGa wt EV RNA 2706")] hr <- hclust(as.dist(1-cor(t(datamat), method="pearson")), method="average") hc <- hclust(as.dist(1-cor(datamat, method="spearman")), method="average") mycl = cutree(hr, h=max(hr$height)/1.05) mycol = c("YELLOW", "DARKBLUE", "DARKORANGE", "DARKMAGENTA", "DARKCYAN", "DARKRED", "MAROON", "DARKGREEN", "LIGHTBLUE", "PINK", "MAGENTA", "LIGHTCYAN","LIGHTGREEN", "BLUE", "ORANGE", "CYAN", "RED", "GREEN"); mycol = mycol[as.vector(mycl)] sampleCols <- rep('GREY',ncol(datamat)) names(sampleCols) <- c("WaGa parental cell RNA","WaGa parental cell RNA 118","WaGa parental cell RNA 147", "WaGa wt EV RNA","WaGa wt EV RNA 2","WaGa wt EV RNA 118","WaGa wt EV RNA 147","WaGa wt EV RNA 226", "WaGa wt EV RNA 1107","WaGa wt EV RNA 1605","WaGa wt EV RNA 2706") #DARKBLUE DARKGREEN DARKRED DARKORANGE sampleCols["WaGa parental cell RNA"] <- 'GREY' sampleCols["WaGa parental cell RNA 118"] <- 'GREY' sampleCols["WaGa parental cell RNA 147"] <- 'GREY' sampleCols["WaGa wt EV RNA"] <- 'GREEN' sampleCols["WaGa wt EV RNA 2"] <- 'GREEN' sampleCols["WaGa wt EV RNA 118"] <- 'GREEN' sampleCols["WaGa wt EV RNA 147"] <- 'GREEN' sampleCols["WaGa wt EV RNA 226"] <- 'GREEN' sampleCols["WaGa wt EV RNA 1107"] <- 'GREEN' sampleCols["WaGa wt EV RNA 1605"] <- 'GREEN' sampleCols["WaGa wt EV RNA 2706"] <- 'GREEN' png("DEGs_Heatmap_WaGa.png", width=1000, height=1200) heatmap.2(as.matrix(datamat),Rowv=as.dendrogram(hr),Colv = NA, dendrogram = 'row', scale='row',trace='none',col=bluered(75), RowSideColors = mycol, ColSideColors = sampleCols, labRow="", margins=c(22,10), cexRow=8, cexCol=2, srtCol=30, lwid=c(1,7), lhei = c(1, 8), legend("top", title = "",legend=c("WaGa parental cell RNA","WaGa wt EV RNA"), fill=c("GREY","GREEN"), cex=1.0, box.lty=0)) dev.off() # save cluster members subset_1<-names(subset(mycl, mycl == '1')) subset_1_ <- getBM(attributes = c('ensembl_gene_id', 'external_gene_name', 'gene_biotype', 'entrezgene_id', 'chromosome_name', 'start_position', 'end_position', 'strand', 'description'), filters = 'ensembl_gene_id', values = subset_1, mart = ensembl) subset_1_uniq <- distinct(subset_1_, ensembl_gene_id, .keep_all= TRUE) subset_1_expr <- datamat[subset_1,] subset_1_expr <- as.data.frame(subset_1_expr) subset_1_expr$ENSEMBL = rownames(subset_1_expr) cluster1_YELLOW <- merge(subset_1_uniq, subset_1_expr, by.x="ensembl_gene_id", by.y="ENSEMBL") #write.csv(cluster1_YELLOW,file='cluster1_YELLOW.txt') subset_2<-names(subset(mycl, mycl == '2')) subset_2_ <- getBM(attributes = c('ensembl_gene_id', 'external_gene_name', 'gene_biotype', 'entrezgene_id', 'chromosome_name', 'start_position', 'end_position', 'strand', 'description'), filters = 'ensembl_gene_id', values = subset_2, mart = ensembl) subset_2_uniq <- distinct(subset_2_, ensembl_gene_id, .keep_all= TRUE) subset_2_expr <- datamat[subset_2,] subset_2_expr <- as.data.frame(subset_2_expr) subset_2_expr$ENSEMBL = rownames(subset_2_expr) cluster2_DARKBLUE <- merge(subset_2_uniq, subset_2_expr, by.x="ensembl_gene_id", by.y="ENSEMBL") #write.csv(cluster2_DARKBLUE,file='cluster2_DARKBLUE.txt') write_xlsx(list( "Cluster 1 YELLOW" = cluster1_YELLOW, "Cluster 2 DARKBLUE" = cluster2_DARKBLUE ), "DEGs_heatmap_data_WaGa.xlsx") # -- MKL-1 cell line -- cut -d',' -f1-1 ./MKL-1_wt.EV_vs_parental-up.txt > MKL-1_wt.EV_vs_parental-up.id cut -d',' -f1-1 ./MKL-1_wt.EV_vs_parental-down.txt > MKL-1_wt.EV_vs_parental-down.id cat MKL-1_wt.EV_vs_parental-up.id MKL-1_wt.EV_vs_parental-down.id | sort -u > ids #20720 #add Gene_Id in the first line. GOI <- read.csv("ids")$Gene_Id RNASeq.NoCellLine <- assay(rld) #clustering methods: "ward.D", "ward.D2", "single", "complete", "average" (= UPGMA), "mcquitty" (= WPGMA), "median" (= WPGMC) or "centroid" (= UPGMC). pearson or spearman datamat = RNASeq.NoCellLine[GOI, c("MKL-1 parental cell RNA","MKL-1 parental cell RNA 118","MKL-1 parental cell RNA 147", "MKL-1 wt EV RNA","MKL-1 wt EV RNA 2","MKL-1 wt EV RNA 27","MKL-1 wt EV RNA 87","MKL-1 wt EV RNA 118", "MKL-1 wt EV RNA 042")] #Check for Zero Variance Rows; If any row has variance 0, remove it. #datamat <- datamat[apply(datamat, 1, var) != 0, ] hr <- hclust(as.dist(1-cor(t(datamat), method="pearson")), method="average") hc <- hclust(as.dist(1-cor(datamat, method="spearman")), method="average") mycl = cutree(hr, h=max(hr$height)/1.05) mycol = c("YELLOW", "DARKBLUE", "DARKORANGE", "DARKMAGENTA", "DARKCYAN", "DARKRED", "MAROON", "DARKGREEN", "LIGHTBLUE", "PINK", "MAGENTA", "LIGHTCYAN","LIGHTGREEN", "BLUE", "ORANGE", "CYAN", "RED", "GREEN"); mycol = mycol[as.vector(mycl)] sampleCols <- rep('GREY',ncol(datamat)) names(sampleCols) <- c("MKL-1 parental cell RNA","MKL-1 parental cell RNA 118","MKL-1 parental cell RNA 147", "MKL-1 wt EV RNA","MKL-1 wt EV RNA 2","MKL-1 wt EV RNA 27","MKL-1 wt EV RNA 87","MKL-1 wt EV RNA 118", "MKL-1 wt EV RNA 042") sampleCols["MKL-1 parental cell RNA"] <- 'GREY' sampleCols["MKL-1 parental cell RNA 118"] <- 'GREY' sampleCols["MKL-1 parental cell RNA 147"] <- 'GREY' sampleCols["MKL-1 wt EV RNA"] <- 'GREEN' sampleCols["MKL-1 wt EV RNA 2"] <- 'GREEN' sampleCols["MKL-1 wt EV RNA 27"] <- 'GREEN' sampleCols["MKL-1 wt EV RNA 87"] <- 'GREEN' sampleCols["MKL-1 wt EV RNA 118"] <- 'GREEN' sampleCols["MKL-1 wt EV RNA 042"] <- 'GREEN' png("DEGs_Heatmap_MKL-1.png", width=1000, height=1200) heatmap.2(as.matrix(datamat),Rowv=as.dendrogram(hr),Colv = NA, dendrogram = 'row', scale='row',trace='none',col=bluered(75), RowSideColors = mycol, ColSideColors = sampleCols, labRow="", margins=c(22,10), cexRow=8, cexCol=2, srtCol=30, lwid=c(1,7), lhei = c(1, 8), legend("top", title = "",legend=c("MKL-1 parental cell RNA","MKL-1 wt EV RNA"), fill=c("GREY","GREEN"), cex=1.0, box.lty=0)) dev.off() # save cluster members subset_1<-names(subset(mycl, mycl == '1')) subset_1_ <- getBM(attributes = c('ensembl_gene_id', 'external_gene_name', 'gene_biotype', 'entrezgene_id', 'chromosome_name', 'start_position', 'end_position', 'strand', 'description'), filters = 'ensembl_gene_id', values = subset_1, mart = ensembl) subset_1_uniq <- distinct(subset_1_, ensembl_gene_id, .keep_all= TRUE) subset_1_expr <- datamat[subset_1,] subset_1_expr <- as.data.frame(subset_1_expr) subset_1_expr$ENSEMBL = rownames(subset_1_expr) cluster1_YELLOW <- merge(subset_1_uniq, subset_1_expr, by.x="ensembl_gene_id", by.y="ENSEMBL") #write.csv(cluster1_YELLOW,file='cluster1_YELLOW.txt') subset_2<-names(subset(mycl, mycl == '2')) subset_2_ <- getBM(attributes = c('ensembl_gene_id', 'external_gene_name', 'gene_biotype', 'entrezgene_id', 'chromosome_name', 'start_position', 'end_position', 'strand', 'description'), filters = 'ensembl_gene_id', values = subset_2, mart = ensembl) subset_2_uniq <- distinct(subset_2_, ensembl_gene_id, .keep_all= TRUE) subset_2_expr <- datamat[subset_2,] subset_2_expr <- as.data.frame(subset_2_expr) subset_2_expr$ENSEMBL = rownames(subset_2_expr) cluster2_DARKBLUE <- merge(subset_2_uniq, subset_2_expr, by.x="ensembl_gene_id", by.y="ENSEMBL") #write.csv(cluster2_DARKBLUE,file='cluster2_DARKBLUE.txt') write_xlsx(list( "Cluster 1 YELLOW" = cluster1_YELLOW, "Cluster 2 DARKBLUE" = cluster2_DARKBLUE ), "DEGs_heatmap_data_MKL-1.xlsx") -
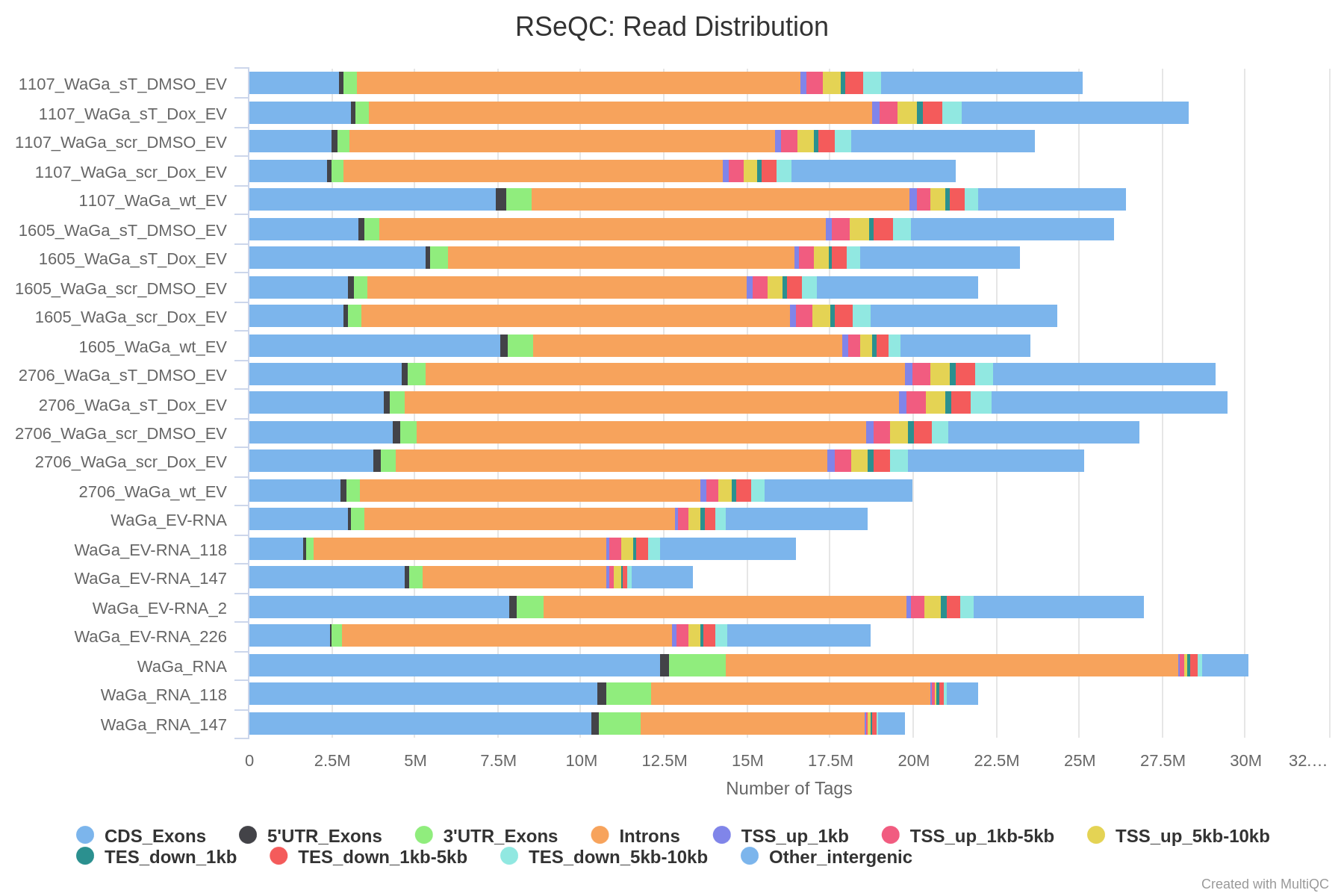
Distribution of different RNA Species untreated/wt and parental; 1x für WaGa cell line und 1x für MKL-1 cells
cd ~/DATA/Data_Ute/Data_RNA-Seq_MKL-1+WaGa/results_2025_1/read_distributions cp ../../../Data_RNA-Seq_WaGa/results/rseqc/read_distribution/WaGa_RNAAligned.sortedByCoord.out.read_distribution.txt WaGa_parental_cell_RNA_read_distribution.txt cp ../../../Data_RNA-Seq_WaGa/results/rseqc/read_distribution/WaGa_RNA_118Aligned.sortedByCoord.out.read_distribution.txt WaGa_parental_cell_RNA_118_read_distribution.txt cp ../../../Data_RNA-Seq_WaGa/results/rseqc/read_distribution/WaGa_RNA_147Aligned.sortedByCoord.out.read_distribution.txt WaGa_parental_cell_RNA_147_read_distribution.txt cp ../../../Data_RNA-Seq_WaGa/results/rseqc/read_distribution/WaGa_EV-RNAAligned.sortedByCoord.out.read_distribution.txt WaGa_wt_EV_RNA_read_distribution.txt cp ../../../Data_RNA-Seq_WaGa/results/rseqc/read_distribution/WaGa_EV-RNA_2Aligned.sortedByCoord.out.read_distribution.txt WaGa_wt_EV_RNA_2_read_distribution.txt cp ../../../Data_RNA-Seq_WaGa/results/rseqc/read_distribution/WaGa_EV-RNA_118Aligned.sortedByCoord.out.read_distribution.txt WaGa_wt_EV_RNA_118_read_distribution.txt cp ../../../Data_RNA-Seq_WaGa/results/rseqc/read_distribution/WaGa_EV-RNA_147Aligned.sortedByCoord.out.read_distribution.txt WaGa_wt_EV_RNA_147_read_distribution.txt cp ../../../Data_RNA-Seq_WaGa/results/rseqc/read_distribution/WaGa_EV-RNA_226Aligned.sortedByCoord.out.read_distribution.txt WaGa_wt_EV_RNA_226_read_distribution.txt cp ../../../Data_RNA-Seq_WaGa/results/rseqc/read_distribution/1107_WaGa_wt_EVAligned.sortedByCoord.out.read_distribution.txt WaGa_wt_EV_RNA_1107_read_distribution.txt cp ../../../Data_RNA-Seq_WaGa/results/rseqc/read_distribution/1605_WaGa_wt_EVAligned.sortedByCoord.out.read_distribution.txt WaGa_wt_EV_RNA_1605_read_distribution.txt cp ../../../Data_RNA-Seq_WaGa/results/rseqc/read_distribution/2706_WaGa_wt_EVAligned.sortedByCoord.out.read_distribution.txt WaGa_wt_EV_RNA_2706_read_distribution.txt cp ../../../Data_RNA-Seq_MKL-1/results/rseqc/read_distribution/MKL-1_RNAAligned.sortedByCoord.out.read_distribution.txt MKL-1_parental_cell_RNA_read_distribution.txt cp ../../../Data_RNA-Seq_MKL-1/results/rseqc/read_distribution/MKL-1_RNA_118Aligned.sortedByCoord.out.read_distribution.txt MKL-1_parental_cell_RNA_118_read_distribution.txt cp ../../../Data_RNA-Seq_MKL-1/results/rseqc/read_distribution/MKL-1_RNA_147Aligned.sortedByCoord.out.read_distribution.txt MKL-1_parental_cell_RNA_147_read_distribution.txt cp ../../../Data_RNA-Seq_MKL-1/results/rseqc/read_distribution/MKL-1_EV-RNAAligned.sortedByCoord.out.read_distribution.txt MKL-1_wt_EV_RNA_read_distribution.txt cp ../../../Data_RNA-Seq_MKL-1/results/rseqc/read_distribution/MKL-1_EV-RNA_2Aligned.sortedByCoord.out.read_distribution.txt MKL-1_wt_EV_RNA_2_read_distribution.txt cp ../../../Data_RNA-Seq_MKL-1/results/rseqc/read_distribution/MKL-1_EV-RNA_27Aligned.sortedByCoord.out.read_distribution.txt MKL-1_wt_EV_RNA_27_read_distribution.txt cp ../../../Data_RNA-Seq_MKL-1/results/rseqc/read_distribution/MKL-1_EV-RNA_87Aligned.sortedByCoord.out.read_distribution.txt MKL-1_wt_EV_RNA_87_read_distribution.txt -
RNA fragmentation patterns: is EV-RNA full length or fragmented?
Since our data consists of single-end RNA-Seq reads, determining the exact RNA fragment length is challenging, as only the read length is available. Unlike paired-end sequencing, where insert size can be calculated, single-end sequencing does not provide direct information about the full fragment size.
点赞本文的读者
还没有人对此文章表态
本文有评论
没有评论
看文章,发评论,不要沉默
最受欢迎文章
- Motif Discovery in Biological Sequences: A Comparison of MEME and HOMER
- Calling peaks using findPeaks of HOMER
- Kraken2 Installation and Usage Guide
- Why Do Significant Gene Lists Change After Adding Additional Conditions in Differential Gene Expression Analysis?
- Should the inputs for GSVA be normalized or raw?
- PiCRUST2 Pipeline for Functional Prediction and Pathway Analysis in Metagenomics
- Updating Human Gene Identifiers using Ensembl BioMart: A Step-by-Step Guide
- pheatmap vs heatmap.2
- Setup conda environments
- Guide to Submitting Data to GEO (Gene Expression Omnibus)
最新文章
- Risks of Rebooting into Rescue Mode
- 足突(Podosome)、胞外囊泡(Extracellular Vesicle)与基质金属蛋白酶(MMPs)综合解析
- NCBI BioSample Submission Strategy for PJI and Nasal Microbiota Study
- Human RNA-seq processing for Data_Ben_RNAseq_2025
最多评论文章
- Updating Human Gene Identifiers using Ensembl BioMart: A Step-by-Step Guide
- The top 10 genes
- Retrieving KEGG Genes Using Bioservices in Python
推荐相似文章
Processing Data_Michelle_RNAseq_2025
Setup the environment for lumicks-pylake and C_Trap-Multimer-photontrack.ipynb
🧬 Cadmium Resistance Gene Analysis in Staphylococcus epidermidis HD46
Workflow for RNA-Binding Protein Enrichment and RNA Type Distribution Analysis (Ute’s Project)